Lithotrophic organisms obtain energy by oxidizing inorganic substances, playing a crucial role in biogeochemical cycles like nitrogen and sulfur transformations. These microbes thrive in diverse environments, from deep-sea vents to soil ecosystems, influencing nutrient availability and ecosystem health. Explore the article to understand how lithotrophic processes impact Your environment and potential applications.
Table of Comparison
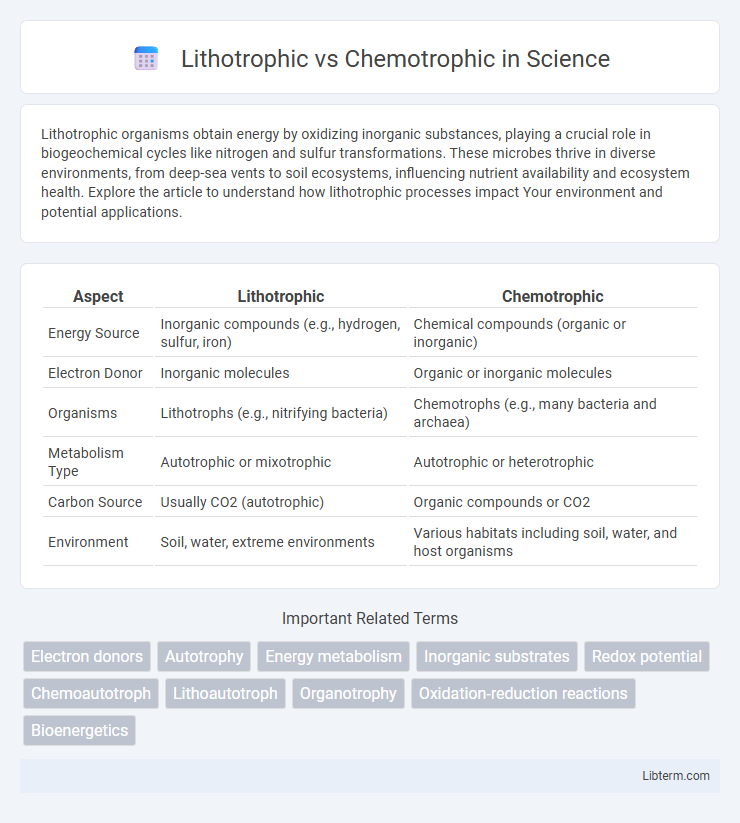
| Aspect | Lithotrophic | Chemotrophic |
|---|---|---|
| Energy Source | Inorganic compounds (e.g., hydrogen, sulfur, iron) | Chemical compounds (organic or inorganic) |
| Electron Donor | Inorganic molecules | Organic or inorganic molecules |
| Organisms | Lithotrophs (e.g., nitrifying bacteria) | Chemotrophs (e.g., many bacteria and archaea) |
| Metabolism Type | Autotrophic or mixotrophic | Autotrophic or heterotrophic |
| Carbon Source | Usually CO2 (autotrophic) | Organic compounds or CO2 |
| Environment | Soil, water, extreme environments | Various habitats including soil, water, and host organisms |
Introduction to Lithotrophic and Chemotrophic Organisms
Lithotrophic organisms obtain energy by oxidizing inorganic substances such as ammonia, hydrogen sulfide, or ferrous iron, playing a crucial role in nutrient cycling and environmental biogeochemical processes. Chemotrophic organisms derive energy from chemical compounds, which can be organic or inorganic, distinguishing them into chemoautotrophs and chemoheterotrophs based on their carbon source. Both lithotrophs and chemotrophs are essential in ecosystems for sustaining microbial metabolism and influencing the global energy flow through diverse metabolic pathways.
Defining Lithotrophy: Energy from Inorganic Sources
Lithotrophy is a metabolic process in which organisms derive energy by oxidizing inorganic compounds such as hydrogen, sulfur, or iron, distinguishing it from chemotrophy that includes both inorganic and organic electron donors. Lithotrophs utilize inorganic molecules like ammonia (NH3), hydrogen gas (H2), or ferrous iron (Fe2+) as electron donors to drive ATP synthesis via electron transport chains. This energy acquisition method enables lithotrophic bacteria and archaea to thrive in environments lacking organic nutrients, making them essential players in biogeochemical cycles.
Understanding Chemotrophy: Energy via Chemical Reactions
Chemotrophy involves organisms obtaining energy by oxidizing chemical compounds, such as hydrogen sulfide or ammonia, rather than relying on sunlight. Lithotrophic organisms, a subset of chemotrophs, specifically use inorganic molecules as their electron donors for energy production. Understanding chemotrophy highlights the diversity of metabolic pathways enabling life to thrive in environments devoid of light, through chemical energy transformations.
Key Differences Between Lithotrophs and Chemotrophs
Lithotrophs derive energy by oxidizing inorganic substances such as ammonia, hydrogen, or iron, distinguishing them from chemotrophs, which obtain energy from chemical compounds, including both organic and inorganic sources. Chemotrophs encompass a broader category, including lithotrophs as well as organotrophs, which metabolize organic compounds for energy. The key difference lies in lithotrophs exclusively using inorganic electron donors, whereas chemotrophs utilize a wider range of chemical substances to generate energy.
Ecological Roles and Habitats
Lithotrophic organisms, which derive energy by oxidizing inorganic compounds such as iron, sulfur, or ammonia, play critical roles in nutrient cycling and biogeochemical processes in environments like deep-sea hydrothermal vents, acidic mine drainages, and soil ecosystems. Chemotrophic organisms encompass both lithotrophs and chemoorganotrophs, utilizing chemical compounds (either inorganic or organic) for energy and occupying diverse habitats including soil, sediments, aquatic environments, and extreme ecosystems. These metabolic strategies support ecological functions such as nitrogen fixation, sulfur oxidation, and carbon cycling, thus sustaining microbial communities and influencing ecosystem productivity.
Metabolic Pathways: Lithotrophs vs Chemotrophs
Lithotrophs utilize inorganic electron donors such as hydrogen, ammonia, or sulfur compounds in their metabolic pathways, fueling energy production primarily through chemolithotrophy. Chemotrophs, on the other hand, derive energy from the oxidation of organic compounds, engaging in chemoorganotrophic metabolism that often involves processes like glycolysis and the citric acid cycle. Both metabolic strategies contribute to ATP synthesis via electron transport chains but differ fundamentally in their electron donor sources and associated biochemical pathways.
Examples of Lithotrophic Organisms
Lithotrophic organisms obtain energy by oxidizing inorganic substances such as hydrogen sulfide, ammonia, or ferrous iron, examples include Nitrosomonas, which oxidizes ammonia, and Acidithiobacillus, known for oxidizing sulfur compounds. Some lithotrophs like Beggiatoa oxidize hydrogen sulfide, contributing to sulfur cycling in aquatic environments. These organisms contrast with chemotrophs that can oxidize organic or inorganic compounds for energy, but lithotrophs specifically rely on inorganic electron donors, playing crucial roles in biogeochemical processes.
Examples of Chemotrophic Organisms
Chemotrophic organisms obtain energy by oxidizing inorganic or organic compounds, with notable examples including Nitrosomonas, which oxidizes ammonia to nitrite, and Sulfolobus, a thermoacidophilic archaeon that oxidizes sulfur compounds in extreme environments. Nitrifying bacteria like Nitrobacter convert nitrite into nitrate, playing a crucial role in the nitrogen cycle. Chemolithoautotrophs such as Hydrogenobacter utilize hydrogen as an energy source, highlighting the diversity and ecological importance of chemotrophic metabolism.
Environmental Impact and Applications
Lithotrophic organisms, which derive energy from inorganic compounds such as sulfur or iron, play a crucial role in biogeochemical cycles by facilitating nutrient recycling and detoxification of pollutants in aquatic and soil environments. Chemotrophic organisms include both lithotrophs and chemoorganotrophs; their metabolic diversity drives applications in bioremediation, wastewater treatment, and bioenergy production due to their ability to metabolize a wide range of chemical substrates. Exploiting these organisms enhances sustainable environmental management by reducing toxic waste accumulation and promoting ecosystem stability.
Conclusion: Significance in Ecosystems and Biotechnology
Lithotrophic organisms obtain energy by oxidizing inorganic substances, playing a crucial role in nutrient cycling and maintaining ecosystem stability. Chemotrophic organisms, which utilize chemical compounds as energy sources, support biodiversity by driving essential biochemical processes in various habitats. Their metabolic diversity has significant biotechnological applications, including bioremediation, bioenergy production, and industrial biosynthesis.
Lithotrophic Infographic
 libterm.com
libterm.com