Alkaliphilic organisms thrive in environments with high pH levels, often above pH 9, showcasing remarkable adaptations that allow their cellular processes to function optimally under alkaline conditions. These microbes play essential roles in industrial applications such as waste treatment and bioremediation, leveraging enzymes stable at high pH for efficient biochemical reactions. Discover how alkaliphiles' unique biology can impact your scientific or industrial endeavors by reading the rest of the article.
Table of Comparison
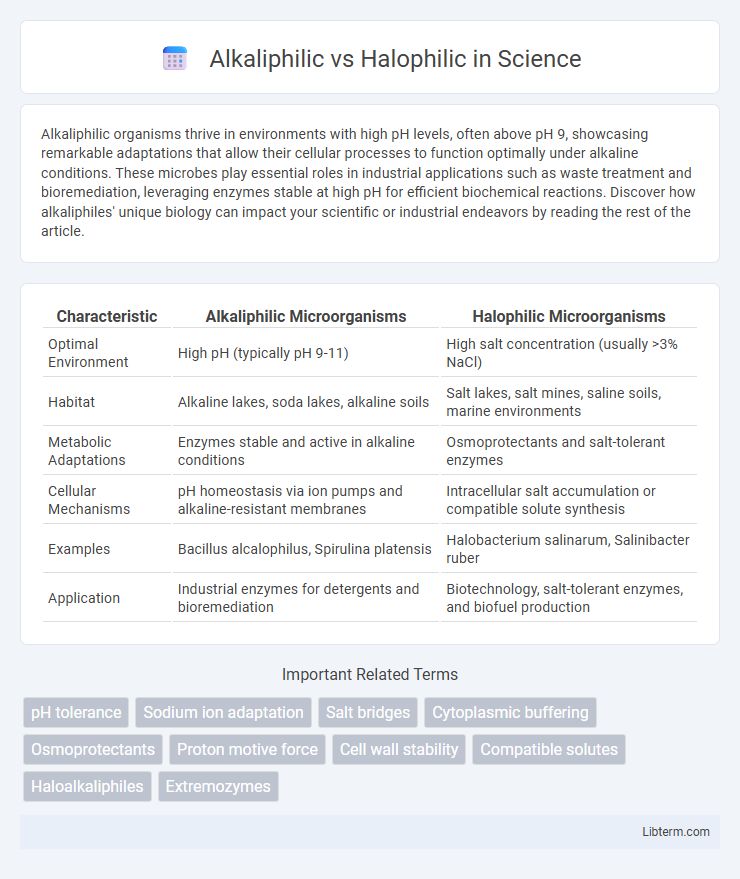
| Characteristic | Alkaliphilic Microorganisms | Halophilic Microorganisms |
|---|---|---|
| Optimal Environment | High pH (typically pH 9-11) | High salt concentration (usually >3% NaCl) |
| Habitat | Alkaline lakes, soda lakes, alkaline soils | Salt lakes, salt mines, saline soils, marine environments |
| Metabolic Adaptations | Enzymes stable and active in alkaline conditions | Osmoprotectants and salt-tolerant enzymes |
| Cellular Mechanisms | pH homeostasis via ion pumps and alkaline-resistant membranes | Intracellular salt accumulation or compatible solute synthesis |
| Examples | Bacillus alcalophilus, Spirulina platensis | Halobacterium salinarum, Salinibacter ruber |
| Application | Industrial enzymes for detergents and bioremediation | Biotechnology, salt-tolerant enzymes, and biofuel production |
Introduction to Extremophiles
Extremophiles thrive in hostile environments, with alkaliphilic microorganisms adapted to highly alkaline conditions above pH 9, and halophilic organisms specialized in high-salt habitats often exceeding 15% NaCl concentration. Both groups possess unique enzymes and cellular mechanisms that maintain stability and functionality under extreme pH or salinity. Studying alkaliphilic and halophilic extremophiles advances biotechnology applications such as bioremediation and industrial enzyme production.
Defining Alkaliphilic and Halophilic Organisms
Alkaliphilic organisms thrive in environments with high pH levels, typically above pH 9, adapting to alkaline conditions by maintaining internal pH homeostasis and specialized enzyme systems. Halophilic organisms require high salt concentrations, often exceeding 3% sodium chloride, to sustain cellular functions and structural stability in hypersaline habitats. Both alkaliphilic and halophilic microbes employ unique physiological mechanisms to survive extreme conditions, highlighting their ecological significance in bioremediation and industrial applications.
Environmental Niches: Alkaline vs Saline Habitats
Alkaliphilic organisms thrive in highly alkaline environments with pH values typically above 9, such as soda lakes and alkaline soils, where they adapt to high hydroxide ion concentrations. Halophilic microorganisms inhabit saline habitats like salt lakes, salt mines, and marine environments with high sodium chloride concentrations, demonstrating specialized mechanisms to maintain cellular osmotic balance. Both groups occupy extreme ecological niches but differ fundamentally in their adaptation to pH versus salinity stresses.
Cellular Adaptations for Survival
Alkaliphilic microorganisms adapt to high pH environments by maintaining intracellular pH homeostasis through specialized ion transporters such as Na+/H+ antiporters and robust cell wall structures that prevent proton leakage. Halophilic organisms survive in high-salinity conditions by accumulating compatible solutes like potassium chloride or synthesizing osmoprotectants, balancing osmotic pressure and stabilizing proteins and membranes. Both adaptations involve intricate cellular mechanisms that optimize enzyme function and structural integrity under extreme environmental stress.
Mechanisms of pH and Salt Tolerance
Alkaliphilic organisms maintain pH homeostasis through specialized sodium/proton antiporters and robust cell wall structures that prevent alkaline damage, enabling survival in environments with pH levels above 9. Halophilic microbes utilize osmoprotectants such as compatible solutes and possess high intracellular concentrations of potassium ions to counteract osmotic stress in hypersaline habitats. Both adaptations involve unique membrane transport systems and enzymatic modifications that optimize cellular function under extreme pH or salt conditions.
Key Differences: Alkaliphiles vs Halophiles
Alkaliphiles thrive in environments with high pH levels, typically above pH 9, whereas halophiles require high salt concentrations, often exceeding 15-20% NaCl, for optimal growth. Alkaliphiles maintain cellular function in alkaline conditions by utilizing specialized ion pumps to regulate intracellular pH, while halophiles accumulate compatible solutes or use ion pumps to balance osmotic pressure in hypersaline habitats. These fundamental adaptations highlight the distinct biochemical and ecological niches of alkaliphiles in alkaline environments versus halophiles in high-salinity ecosystems.
Ecological Roles and Distribution
Alkaliphilic microorganisms thrive in high pH environments such as soda lakes and alkaline soils, playing crucial roles in nutrient cycling, organic matter decomposition, and bioremediation in these extreme habitats. Halophilic microorganisms inhabit high-salinity environments like salt flats, saline lakes, and marine salterns, contributing to the carbon and nitrogen cycles and supporting unique microbial communities adapted to osmotic stress. Both groups are widely distributed globally but occupy distinct ecological niches shaped by pH and salinity gradients, influencing ecosystem stability and functionality in extreme environments.
Industrial and Biotechnological Applications
Alkaliphilic microorganisms thrive in high pH environments, making them valuable for industrial processes such as detergent formulation, bioremediation, and enzyme production like proteases and amylases that function optimally in alkaline conditions. Halophilic microbes, adapted to high-salt environments, are exploited in biotechnology for producing stable enzymes, bioplastics, and bioactive compounds used in pharmaceuticals and food preservation. Industrial applications leverage the unique stress tolerance of alkaliphilic and halophilic enzymes to enhance efficiency, stability, and cost-effectiveness in harsh processing conditions.
Challenges in Cultivation and Study
Cultivating alkaliphilic microorganisms requires maintaining high pH environments, often above pH 9, which can destabilize standard culture media and inhibit growth of non-adapted species, posing significant laboratory challenges. Halophilic microorganisms demand elevated salt concentrations, typically above 3% NaCl, causing osmotic stress and requiring specialized media formulations to prevent plasmolysis or enzyme inactivation. Both groups present difficulties in replicating their natural extremophile conditions, limiting the understanding of their metabolic pathways, genetic regulation, and potential biotechnological applications.
Future Perspectives in Extremophile Research
Future perspectives in extremophile research emphasize the potential of alkaliphilic and halophilic microorganisms for biotechnological innovation, especially in industrial enzyme production and bioremediation under extreme conditions. Advances in genomics and synthetic biology enable the design of tailored microbial strains capable of thriving in high-pH and high-salinity environments, expanding applications in pharmaceuticals and bioenergy. Understanding their unique metabolic pathways also offers insights into astrobiology and the search for life in extraterrestrial habitats characterized by extreme alkalinity and salinity.
Alkaliphilic Infographic
 libterm.com
libterm.com