Van der Waals forces are weak intermolecular attractions that play a crucial role in the physical properties of molecules, including boiling points and solubility. These forces influence how molecules interact in gases, liquids, and solids, affecting phenomena like adhesion and cohesion. Explore the rest of the article to understand how Van der Waals forces impact your everyday life and scientific applications.
Table of Comparison
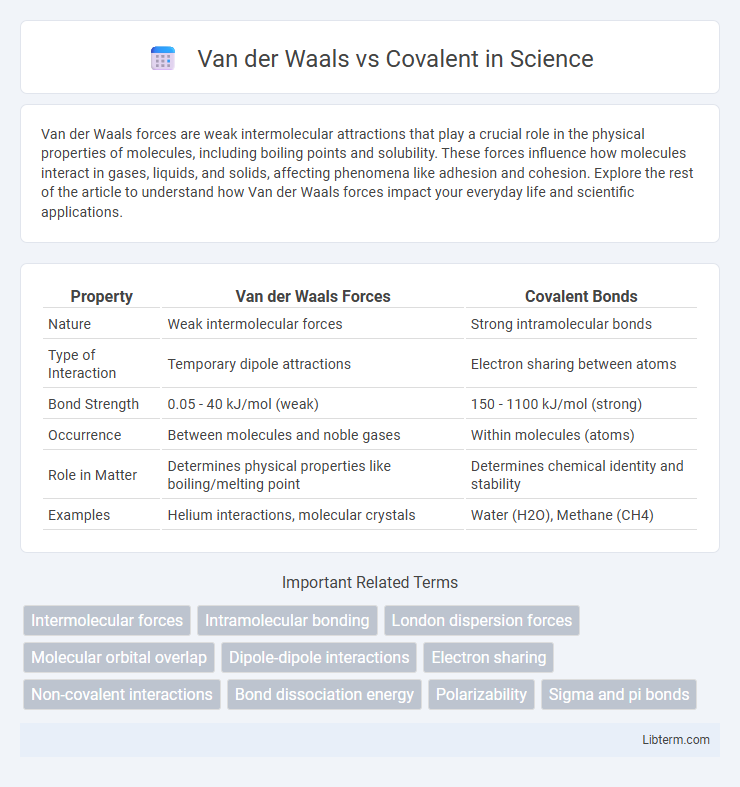
| Property | Van der Waals Forces | Covalent Bonds |
|---|---|---|
| Nature | Weak intermolecular forces | Strong intramolecular bonds |
| Type of Interaction | Temporary dipole attractions | Electron sharing between atoms |
| Bond Strength | 0.05 - 40 kJ/mol (weak) | 150 - 1100 kJ/mol (strong) |
| Occurrence | Between molecules and noble gases | Within molecules (atoms) |
| Role in Matter | Determines physical properties like boiling/melting point | Determines chemical identity and stability |
| Examples | Helium interactions, molecular crystals | Water (H2O), Methane (CH4) |
Introduction to Van der Waals and Covalent Bonds
Van der Waals forces are weak intermolecular attractions arising from temporary dipoles induced in atoms or molecules, playing a crucial role in the physical properties of noble gases and molecular solids. Covalent bonds involve the sharing of electron pairs between atoms, resulting in strong and directional connections fundamental to the structure of molecules like water, methane, and DNA. Understanding the distinction between Van der Waals interactions and covalent bonds is essential for grasping molecular behavior, chemical reactivity, and material characteristics.
Defining Van der Waals Forces
Van der Waals forces are weak intermolecular attractions arising from temporary dipoles induced in atoms or molecules due to fluctuating electron distributions. These forces differ from covalent bonds, which involve the sharing of electron pairs between atoms to form strong, stable chemical bonds. Van der Waals interactions play a crucial role in molecular recognition, surface adhesion, and the physical properties of nonpolar compounds.
Understanding Covalent Bonds
Covalent bonds form when atoms share pairs of electrons to achieve stable electron configurations, typically between nonmetal atoms, resulting in strong, directional bonds. These bonds create distinct molecules with specific shapes defined by the overlap of atomic orbitals and electron pair distributions. Understanding covalent bonding involves recognizing bond polarity, bond energy, and bond length, which influence molecular properties and reactivity.
Key Differences Between the Two Bonds
Van der Waals forces are weak intermolecular attractions resulting from temporary dipoles, whereas covalent bonds involve the sharing of electron pairs between atoms, creating strong intramolecular connections. Van der Waals interactions primarily influence physical properties like boiling points and melting points, while covalent bonds determine molecular structure and chemical reactivity. Van der Waals forces vary in strength depending on molecular size and shape, contrasting with the fixed bond energy characteristic of covalent bonds.
Molecular Structure and Bond Strength
Van der Waals forces are weak intermolecular attractions caused by temporary dipoles in molecules, resulting in low bond strength and flexible, easily disrupted molecular structures. Covalent bonds involve the sharing of electron pairs between atoms, creating strong, stable bonds that define the fixed geometry and rigidity of molecular structures. Molecular structures with covalent bonding exhibit higher bond dissociation energy compared to those dominated by Van der Waals interactions.
Examples in Everyday Life
Van der Waals forces are responsible for the adhesion of gecko feet to walls and the attraction between water molecules that enable surface tension in liquids. Covalent bonds, seen in everyday substances like water (H2O) and carbon dioxide (CO2), involve the sharing of electron pairs between atoms, providing structural stability to molecules. Common household materials like plastic and proteins owe their durability to covalent bonding, while Van der Waals interactions influence the behavior of gases and the sticking of dust particles on surfaces.
Role in Chemical and Biological Systems
Van der Waals forces play a critical role in the stabilization of biomolecular structures by facilitating weak, non-covalent interactions such as those between protein side chains and lipid membranes, which are essential for molecular recognition and cellular signaling. Covalent bonds provide strong, stable linkages that form the primary structural framework of molecules including DNA, enzymes, and metabolic substrates, ensuring the integrity and functionality of chemical compounds within biological systems. The interplay between covalent bonding and Van der Waals interactions enables dynamic biological processes like protein folding, ligand binding, and enzyme catalysis, highlighting their complementary roles in maintaining life at the molecular level.
Physical Properties Influenced by Each Bond
Van der Waals forces result in substances with low melting and boiling points, soft textures, and poor electrical conductivity due to their weak intermolecular attraction. Covalent bonds create materials with higher melting and boiling points, greater hardness, and variable electrical conductivity, stemming from the strong sharing of electrons between atoms. The differences in bond strength directly influence the thermal stability and mechanical properties of substances.
Applications in Materials Science
Van der Waals forces enable the development of layered materials like graphene and transition metal dichalcogenides, promoting innovations in flexible electronics and nanoscale devices. Covalent bonds provide structural integrity in polymers and ceramics, crucial for creating materials with high strength, thermal stability, and chemical resistance. Understanding the interplay between Van der Waals interactions and covalent bonding allows materials scientists to engineer composites with tailored mechanical and electronic properties.
Conclusion: Which Bond Type Matters Most?
Covalent bonds fundamentally determine the molecular structure and chemical properties by sharing electron pairs between atoms, resulting in strong and stable connections. Van der Waals forces, although weaker and temporary, play a crucial role in intermolecular interactions, influencing physical properties like boiling points and molecular recognition. The importance of each bond type depends on the context: covalent bonds dominate chemical identity and reactivity, while Van der Waals forces govern physical state and molecular assembly.
Van der Waals Infographic
 libterm.com
libterm.com