Recombinant technology involves combining DNA from different sources to create new genetic combinations with desired traits. This method is widely used in medicine, agriculture, and biotechnology to produce proteins, vaccines, and genetically modified organisms. Explore the rest of this article to understand how recombinant techniques can impact your field or interests.
Table of Comparison
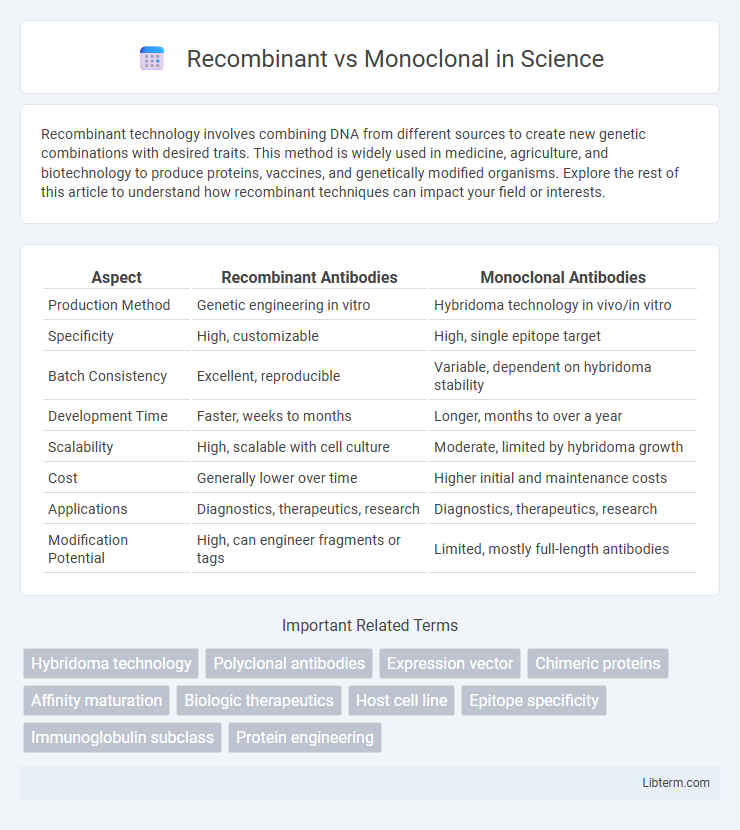
| Aspect | Recombinant Antibodies | Monoclonal Antibodies |
|---|---|---|
| Production Method | Genetic engineering in vitro | Hybridoma technology in vivo/in vitro |
| Specificity | High, customizable | High, single epitope target |
| Batch Consistency | Excellent, reproducible | Variable, dependent on hybridoma stability |
| Development Time | Faster, weeks to months | Longer, months to over a year |
| Scalability | High, scalable with cell culture | Moderate, limited by hybridoma growth |
| Cost | Generally lower over time | Higher initial and maintenance costs |
| Applications | Diagnostics, therapeutics, research | Diagnostics, therapeutics, research |
| Modification Potential | High, can engineer fragments or tags | Limited, mostly full-length antibodies |
Introduction to Recombinant and Monoclonal Technologies
Recombinant technology involves the manipulation of DNA sequences to produce proteins or antibodies by inserting genes into host cells, enabling large-scale production of biologically active molecules. Monoclonal technology refers to the generation of identical antibodies from a single B-cell clone, providing high specificity for targeting antigens in research and therapeutics. Both technologies leverage biotechnology advances to enhance disease diagnosis, treatment, and biological research with precision and efficiency.
Defining Recombinant Proteins
Recombinant proteins are produced by inserting a target gene into host cells, enabling large-scale protein expression with high purity and consistency. Monoclonal proteins, specifically antibodies, are derived from a single clone of cells, ensuring uniform specificity and affinity for a particular antigen. Recombinant technology enhances monoclonal protein production by allowing genetic modification for improved stability and therapeutic efficacy.
Understanding Monoclonal Antibodies
Monoclonal antibodies are identical immunoglobulins produced by a single clone of B cells, designed to bind specifically to a unique antigen epitope, enabling targeted therapies in oncology and autoimmune diseases. Recombinant antibody technology enhances monoclonal antibody production by utilizing genetic engineering to express antibody fragments with improved affinity, stability, and reduced immunogenicity. These advancements facilitate the development of highly specific therapeutic agents and diagnostic tools with greater efficacy and safety profiles.
Production Methods: Recombinant vs Monoclonal
Recombinant antibody production involves inserting specific antibody genes into host cells like bacteria, yeast, or mammalian cells to express the antibody proteins through genetic engineering techniques. Monoclonal antibodies are generated by fusing a single B-cell from an immunized animal with myeloma cells to create hybridomas that produce identical antibody clones in vitro. Recombinant methods offer precise genetic control and scalability, whereas monoclonal production relies on hybridoma technology for stable, consistent antibody generation.
Key Differences in Applications
Recombinant antibodies are engineered through gene cloning techniques, offering high specificity and uniformity for diagnostic and therapeutic applications, including targeted drug delivery and biosensors. Monoclonal antibodies, produced by hybridoma technology, provide consistent antigen specificity, primarily used in clinical diagnostics, cancer therapy, and autoimmune disease treatment. The key difference lies in recombinant antibodies' flexibility for modification and scalability, whereas monoclonal antibodies excel in standardized bulk production and established clinical use.
Advantages of Recombinant Technology
Recombinant technology offers enhanced precision in producing monoclonal antibodies with consistent quality and reduced batch-to-batch variability compared to traditional hybridoma methods. This technology enables large-scale production, increased yield, and the ability to engineer antibodies with improved specificity and reduced immunogenicity. The use of recombinant platforms supports faster development cycles and customization for therapeutic applications, making it a preferred choice in biotechnology and pharmaceutical industries.
Benefits of Monoclonal Antibodies
Monoclonal antibodies offer precise targeting of specific antigens, enhancing diagnostic accuracy and therapeutic efficacy compared to recombinant antibodies. Their uniformity and consistent affinity enable reproducible results in clinical and research applications, reducing variability. These antibodies also facilitate novel treatments and personalized medicine by enabling selective immune modulation and therapeutic intervention.
Challenges and Limitations
Recombinant and monoclonal antibodies face distinct challenges, with recombinant antibodies often limited by their expression yield, stability, and post-translational modifications, impacting their efficacy and scalability. Monoclonal antibodies encounter limitations including potential immunogenicity, high production costs, and difficulties in achieving consistent batch-to-batch quality. Both types require rigorous optimization to overcome issues related to specificity, affinity, and manufacturing reproducibility.
Clinical and Research Implications
Recombinant antibodies offer enhanced specificity and batch-to-batch consistency, making them ideal for precise diagnostic applications and reproducible research outcomes. Monoclonal antibodies, produced via hybridoma technology, provide targeted therapeutic options with proven clinical efficacy but may exhibit variability due to biological production processes. The choice between recombinant and monoclonal antibodies significantly impacts drug development pipelines, immunoassay reliability, and translational research efficacy.
Future Trends and Innovations
Recombinant antibody technology is expected to advance through enhanced engineering techniques, enabling the development of more specific and high-affinity monoclonal antibodies with reduced immunogenicity. Innovations in synthetic biology and machine learning algorithms are driving the creation of next-generation monoclonal antibodies that target complex antigens and improve therapeutic efficacy. Future trends include integration of bispecific and multispecific recombinant antibodies, expanding applications in cancer immunotherapy and personalized medicine.
Recombinant Infographic
 libterm.com
libterm.com