Ferroptosis is a regulated form of cell death driven by iron-dependent lipid peroxidation, distinct from apoptosis and necrosis. This process plays a critical role in various diseases, including cancer, neurodegeneration, and ischemia-reperfusion injury. Discover more about how ferroptosis impacts health and potential therapeutic strategies in the rest of this article.
Table of Comparison
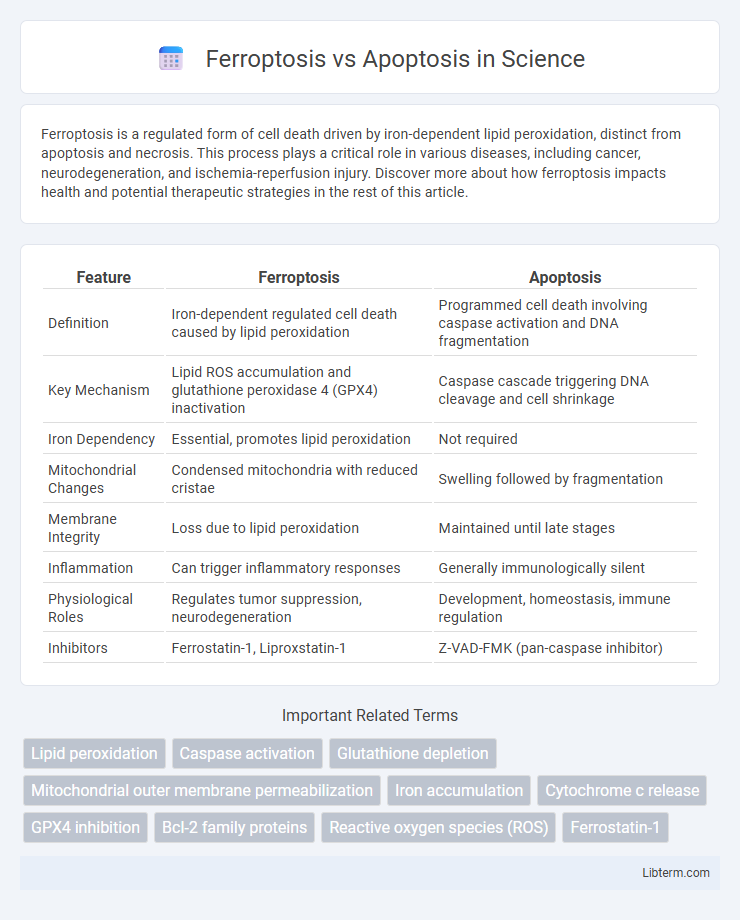
| Feature | Ferroptosis | Apoptosis |
|---|---|---|
| Definition | Iron-dependent regulated cell death caused by lipid peroxidation | Programmed cell death involving caspase activation and DNA fragmentation |
| Key Mechanism | Lipid ROS accumulation and glutathione peroxidase 4 (GPX4) inactivation | Caspase cascade triggering DNA cleavage and cell shrinkage |
| Iron Dependency | Essential, promotes lipid peroxidation | Not required |
| Mitochondrial Changes | Condensed mitochondria with reduced cristae | Swelling followed by fragmentation |
| Membrane Integrity | Loss due to lipid peroxidation | Maintained until late stages |
| Inflammation | Can trigger inflammatory responses | Generally immunologically silent |
| Physiological Roles | Regulates tumor suppression, neurodegeneration | Development, homeostasis, immune regulation |
| Inhibitors | Ferrostatin-1, Liproxstatin-1 | Z-VAD-FMK (pan-caspase inhibitor) |
Introduction to Cell Death Mechanisms
Ferroptosis and apoptosis are distinct cell death mechanisms critical for tissue homeostasis and disease progression. Apoptosis is a programmed, caspase-dependent process characterized by DNA fragmentation and membrane blebbing, while ferroptosis is an iron-dependent form of cell death driven by lipid peroxidation and reactive oxygen species accumulation. Understanding the molecular pathways of ferroptosis and apoptosis provides insights into therapeutic strategies for cancer, neurodegeneration, and ischemia-reperfusion injuries.
Defining Ferroptosis: Key Features
Ferroptosis is a regulated cell death characterized by iron-dependent lipid peroxidation, distinct from apoptosis, which involves caspase-mediated DNA fragmentation. Key features of ferroptosis include the accumulation of reactive oxygen species (ROS), depletion of glutathione, and inactivation of glutathione peroxidase 4 (GPX4). Unlike apoptosis, ferroptosis does not exhibit chromatin condensation or apoptotic body formation but is marked by mitochondrial shrinkage and increased membrane density.
Apoptosis Explained: Classic Pathways
Apoptosis is a programmed cell death mechanism involving intrinsic and extrinsic pathways regulated by proteins such as caspases, Bcl-2 family members, and death receptors. The intrinsic pathway is triggered by mitochondrial outer membrane permeabilization, releasing cytochrome c and activating caspase-9, while the extrinsic pathway involves ligand binding to death receptors like Fas, leading to caspase-8 activation. These pathways ensure controlled cellular dismantling and prevent inflammatory responses, differentiating apoptosis from ferroptosis, which is characterized by iron-dependent lipid peroxidation and oxidative damage.
Molecular Triggers of Ferroptosis
Ferroptosis is triggered by the accumulation of iron-dependent lipid peroxides, primarily driven by the failure of glutathione peroxidase 4 (GPX4) to reduce lipid hydroperoxides, leading to membrane damage. Molecular triggers include the depletion of glutathione, inhibition of cystine uptake via the system Xc- transporter, and increased intracellular free iron through ferritin degradation or transferrin receptor upregulation. Unlike apoptosis, which is initiated by caspase activation and mitochondrial outer membrane permeabilization, ferroptosis relies on iron metabolism dysregulation and oxidative stress pathways.
Apoptosis Initiation and Regulation
Apoptosis initiation involves an intrinsic pathway triggered by mitochondrial outer membrane permeabilization and an extrinsic pathway activated through death receptors like Fas. Key regulators include caspases, Bcl-2 family proteins, and cytochrome c, which facilitate controlled cell dismantling. In contrast to ferroptosis, apoptosis maintains cellular homeostasis through energy-dependent processes that ensure orderly cell death without inducing inflammation.
Distinct Biochemical Markers
Ferroptosis is characterized by iron-dependent lipid peroxidation and the depletion of glutathione peroxidase 4 (GPX4), whereas apoptosis features caspase activation, DNA fragmentation, and phosphatidylserine externalization. Ferroptosis involves accumulation of reactive oxygen species (ROS) specifically in cell membranes, distinct from the mitochondrial outer membrane permeabilization seen in apoptosis. The presence of specific markers such as malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) indicate ferroptosis, contrasting with cleaved poly(ADP-ribose) polymerase (PARP) and cytochrome c release signaling apoptosis.
Morphological Differences: Ferroptosis vs Apoptosis
Ferroptosis is characterized by small mitochondria with condensed membrane densities, reduced or vanished cristae, and ruptured outer membranes, whereas apoptosis shows cell shrinkage, chromatin condensation, and formation of apoptotic bodies. Ferroptotic cells lack the nuclear fragmentation typical of apoptotic cells, and their plasma membranes remain intact until late stages. These distinct morphological features are critical for differentiating ferroptosis from apoptosis in cellular death studies.
Physiological and Pathological Roles
Ferroptosis is a regulated cell death mechanism driven by iron-dependent lipid peroxidation, playing critical roles in neurodegenerative diseases, ischemia-reperfusion injury, and cancer suppression by eliminating damaged cells. Apoptosis, characterized by caspase activation and DNA fragmentation, is essential for normal development, immune system homeostasis, and preventing tumor formation by removing dysfunctional or harmful cells. Dysregulation of ferroptosis contributes to pathological conditions like neurodegeneration and organ damage, whereas defective apoptosis is implicated in cancer progression and autoimmune disorders.
Therapeutic Implications in Disease
Ferroptosis and apoptosis represent distinct cell death pathways with critical therapeutic implications in diseases like cancer and neurodegeneration. Targeting ferroptosis through iron modulation and lipid peroxidation inhibitors offers novel strategies for resistant tumors, while apoptosis-based therapies focus on activating caspases and restoring apoptotic signaling. Understanding the differential molecular mechanisms of these pathways enables precision medicine approaches for treating diseases with dysregulated cell death.
Future Directions in Cell Death Research
Ferroptosis and apoptosis represent distinct, regulated pathways of cell death with unique molecular mechanisms and implications for disease treatment. Future research aims to elucidate the crosstalk between these pathways, identify novel biomarkers for early detection, and develop targeted therapeutics that exploit ferroptotic and apoptotic processes in cancer, neurodegeneration, and other pathologies. Advanced omics technologies and high-throughput screening are expected to accelerate the discovery of modulators that can precisely control cell fate decisions for improved clinical outcomes.
Ferroptosis Infographic
 libterm.com
libterm.com