An enantiomer is one of two stereoisomers that are mirror images of each other but cannot be superimposed, much like left and right hands. These molecules often exhibit different behaviors in biological systems, influencing drug efficacy and safety. Discover how understanding enantiomers can impact your approach to chemistry and pharmaceuticals throughout this article.
Table of Comparison
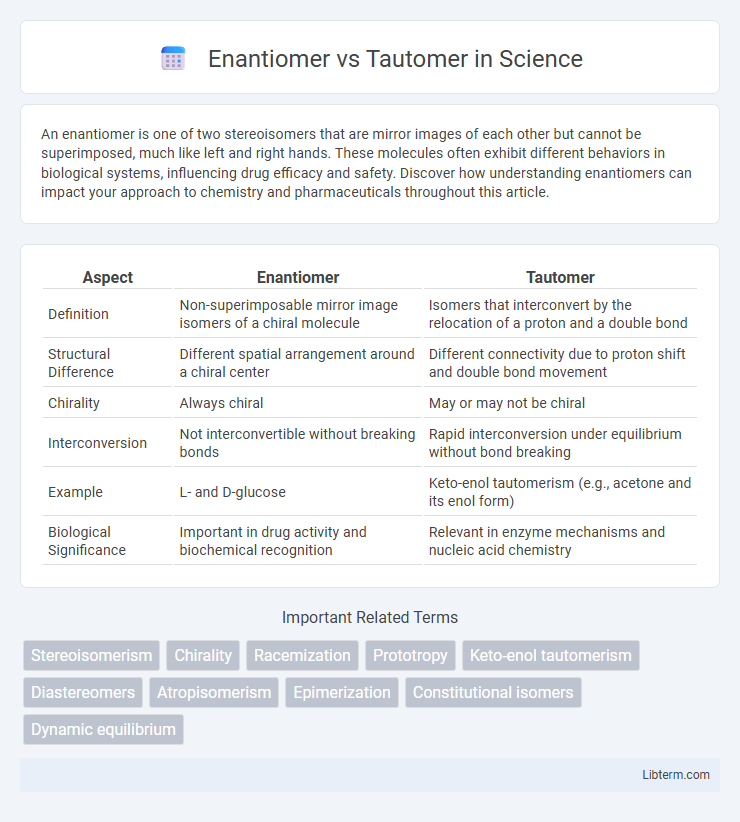
| Aspect | Enantiomer | Tautomer |
|---|---|---|
| Definition | Non-superimposable mirror image isomers of a chiral molecule | Isomers that interconvert by the relocation of a proton and a double bond |
| Structural Difference | Different spatial arrangement around a chiral center | Different connectivity due to proton shift and double bond movement |
| Chirality | Always chiral | May or may not be chiral |
| Interconversion | Not interconvertible without breaking bonds | Rapid interconversion under equilibrium without bond breaking |
| Example | L- and D-glucose | Keto-enol tautomerism (e.g., acetone and its enol form) |
| Biological Significance | Important in drug activity and biochemical recognition | Relevant in enzyme mechanisms and nucleic acid chemistry |
Introduction to Enantiomers and Tautomers
Enantiomers are stereoisomers that are non-superimposable mirror images of each other, differing in the spatial arrangement of atoms around a chiral center. Tautomers are isomers that readily interconvert through the relocation of a proton and a shift in bonding electrons, commonly seen in keto-enol tautomerism. Both enantiomers and tautomers play crucial roles in organic chemistry, influencing molecular behavior, reactivity, and biological activity.
Defining Enantiomers: Structure and Properties
Enantiomers are stereoisomers that are non-superimposable mirror images of each other, differing in the spatial arrangement of atoms around a chiral center, typically a carbon atom with four distinct substituents. These molecules exhibit identical physical properties except for the direction in which they rotate plane-polarized light and their interactions with other chiral environments, such as enzymes or receptors. The specific three-dimensional configuration gives rise to distinct biological activities and pharmacological effects, crucial in drug design and biochemical processes.
Understanding Tautomers: Types and Characteristics
Tautomers are isomers that differ mainly in the position of a proton and the double bond, exhibiting rapid interconversion. The primary types of tautomers include keto-enol and imine-enamine tautomers, each characterized by unique chemical environments and stability profiles. Understanding tautomerism is crucial in pharmaceutical chemistry, as tautomeric forms influence drug efficacy, solubility, and binding interactions.
Key Differences Between Enantiomers and Tautomers
Enantiomers are stereoisomers that are non-superimposable mirror images of each other, differing in the spatial arrangement of atoms but having the same connectivity, while tautomers are structural isomers that interconvert through the movement of a proton and a shift of a double bond, resulting in different connectivity. Enantiomers exhibit identical physical properties except for the direction they rotate plane-polarized light and their interaction with chiral environments, whereas tautomers possess distinct physical and chemical properties due to differences in bonding and atomic arrangement. Enantiomeric relationships are crucial in stereochemistry and chiral drug design, whereas tautomerism plays a significant role in chemical reactivity, enzyme catalysis, and molecular stability.
Molecular Formula and Structure Comparisons
Enantiomers share the same molecular formula and atomic connectivity but differ in the spatial arrangement of their atoms, making them non-superimposable mirror images. Tautomers also have identical molecular formulas but differ in the connectivity of atoms, typically involving the shift of a hydrogen atom and a double bond, resulting in distinct structural isomers. The key distinction lies in enantiomers' stereoisomerism versus tautomers' dynamic structural isomerism due to proton transfer.
Chirality and Optical Activity in Enantiomers
Enantiomers are stereoisomers that are non-superimposable mirror images of each other, exhibiting chirality and resulting in distinct optical activity by rotating plane-polarized light in opposite directions. Tautomers, on the other hand, are structural isomers that interconvert through the relocation of a proton and a double bond, lacking inherent chirality and thus do not display optical activity. Chirality in enantiomers is a critical factor in pharmaceuticals, influencing drug efficacy and interactions due to their specific three-dimensional arrangements.
Proton Transfer and Equilibrium in Tautomers
Tautomers exist in a dynamic equilibrium characterized by proton transfer, often involving the migration of a proton between different atoms within a molecule, resulting in distinct isomeric forms such as keto and enol tautomers. This proton transfer process leads to an equilibrium state where the relative concentrations of tautomers depend on factors like solvent, temperature, and pH. Enantiomers, unlike tautomers, are stereoisomers related by mirror images and do not interconvert through proton transfer, maintaining fixed spatial configurations without dynamic equilibrium between forms.
Importance in Pharmaceuticals and Drug Design
Enantiomers exhibit distinct pharmacological effects due to their mirror-image molecular structures, which interact differently with biological targets, making stereochemistry crucial in drug design for efficacy and safety. Tautomers influence drug stability, bioavailability, and receptor binding through dynamic structural shifts, impacting drug formulation and metabolism. Understanding both enantiomerism and tautomerism enables precise optimization of therapeutic agents, reducing side effects and improving target specificity in pharmaceutical development.
Analytical Techniques for Identification
Enantiomers are often identified using chiral chromatography and circular dichroism spectroscopy, which exploit their optical activity and chiral-specific interactions. Tautomers, due to their rapid interconversion, require techniques like nuclear magnetic resonance (NMR) spectroscopy and infrared (IR) spectroscopy to detect structural differences at the molecular level. Mass spectrometry coupled with chromatographic separation can also help distinguish tautomers by analyzing their unique fragmentation patterns and retention times.
Summary and Scientific Implications
Enantiomers are stereoisomers that are non-superimposable mirror images of each other, impacting drug efficacy and receptor binding specificity due to their chiral nature. Tautomers are isomers that rapidly interconvert by the relocation of a proton and a double bond, influencing chemical reactivity, stability, and bioavailability of compounds. Understanding enantiomeric purity and tautomeric equilibria is crucial in pharmaceutical development and medicinal chemistry to optimize therapeutic outcomes and minimize side effects.
Enantiomer Infographic
 libterm.com
libterm.com