Positron emission is a type of radioactive decay in which a proton inside an unstable nucleus is converted into a neutron, releasing a positron and a neutrino. This process plays a crucial role in medical imaging techniques like PET scans, enabling the visualization of metabolic processes in the body. Explore the rest of the article to understand how positron emission impacts medical diagnostics and research.
Table of Comparison
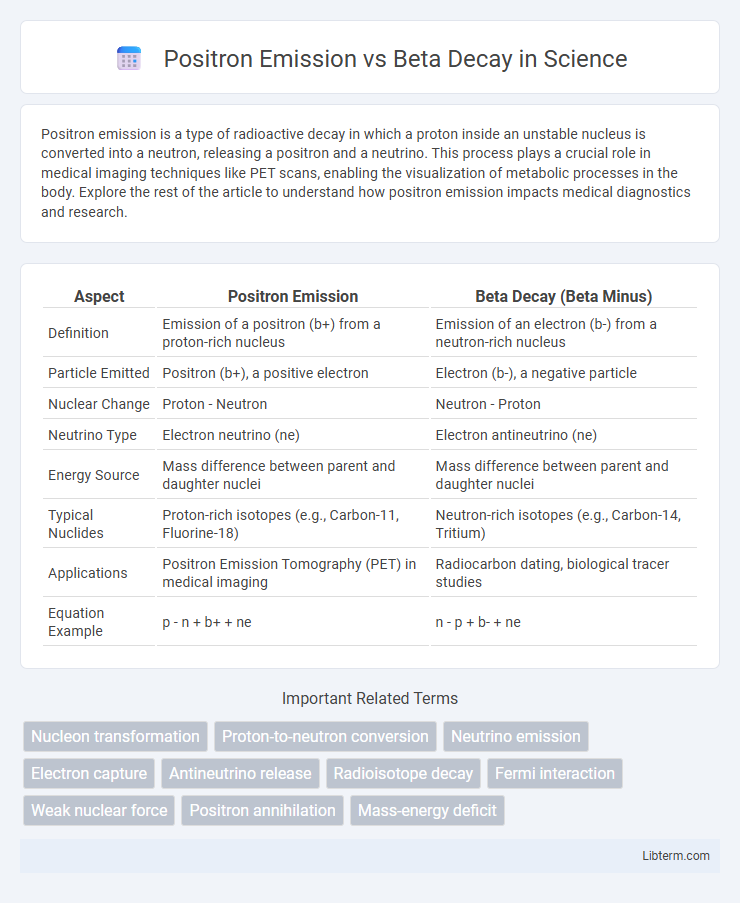
| Aspect | Positron Emission | Beta Decay (Beta Minus) |
|---|---|---|
| Definition | Emission of a positron (b+) from a proton-rich nucleus | Emission of an electron (b-) from a neutron-rich nucleus |
| Particle Emitted | Positron (b+), a positive electron | Electron (b-), a negative particle |
| Nuclear Change | Proton - Neutron | Neutron - Proton |
| Neutrino Type | Electron neutrino (ne) | Electron antineutrino (ne) |
| Energy Source | Mass difference between parent and daughter nuclei | Mass difference between parent and daughter nuclei |
| Typical Nuclides | Proton-rich isotopes (e.g., Carbon-11, Fluorine-18) | Neutron-rich isotopes (e.g., Carbon-14, Tritium) |
| Applications | Positron Emission Tomography (PET) in medical imaging | Radiocarbon dating, biological tracer studies |
| Equation Example | p - n + b+ + ne | n - p + b- + ne |
Understanding Nuclear Decay: An Overview
Positron emission and beta decay are two types of nuclear decay processes that involve the transformation of a neutron into a proton or vice versa, changing the atomic number of an element. In positron emission, a proton is converted into a neutron while emitting a positron and a neutrino, typically occurring in proton-rich nuclei to achieve stability. Beta decay includes both beta-minus decay, where a neutron decays into a proton emitting an electron and an antineutrino, and positron emission, crucial for understanding nuclear stability and the mechanisms governing radioactive decay.
What is Positron Emission?
Positron emission is a type of radioactive decay in which a proton inside an unstable atomic nucleus is converted into a neutron, releasing a positron and a neutrino. This process decreases the atomic number by one while keeping the mass number constant, resulting in the formation of a new element. Positron emission is commonly observed in proton-rich nuclei and is utilized in medical imaging techniques such as positron emission tomography (PET).
Defining Beta Decay: Beta-minus and Beta-plus
Beta decay involves the transformation of a neutron into a proton or vice versa within an atomic nucleus, resulting in the emission of beta particles. Beta-minus decay (b-) emits an electron and an antineutrino when a neutron converts into a proton, while beta-plus decay (b+) or positron emission releases a positron and a neutrino as a proton transforms into a neutron. These processes alter the atomic number and help in nuclear transmutation without changing the mass number.
Atomic Nucleus Changes During Emission
Positron emission involves the conversion of a proton into a neutron within the atomic nucleus, resulting in the release of a positron and a neutrino, which decreases the atomic number by one while keeping the mass number constant. Beta decay, specifically beta minus decay, transforms a neutron into a proton, emitting an electron and an antineutrino, thus increasing the atomic number by one with no change in mass number. Both processes alter the composition of the nucleus by changing the ratio of protons to neutrons, impacting the element's identity but preserving overall nuclear stability.
Fundamental Differences: Positron Emission vs Beta Decay
Positron emission, or beta-plus decay, involves a proton in the nucleus transforming into a neutron while emitting a positron and a neutrino, decreasing the atomic number by one. Beta decay specifically refers to beta-minus decay where a neutron converts into a proton, emitting an electron and an antineutrino, increasing the atomic number by one. The fundamental difference lies in particle emission and nuclear transformation: positron emission reduces proton count, whereas beta-minus decay increases it.
Particle Emission: Positrons vs Electrons
Positron emission involves the release of a positron, the antimatter counterpart of the electron, from a proton-rich nucleus, while beta decay typically emits an electron or beta particle from a neutron-rich nucleus. Positrons carry a positive charge (+1), resulting in their annihilation with electrons upon interaction, producing gamma rays, whereas beta particles (electrons) have a negative charge (-1) and penetrate matter differently. The distinction between these particle emissions is crucial in medical imaging, such as positron emission tomography (PET), and nuclear physics applications.
Energy Considerations in Nuclear Transmutation
Positron emission involves a proton in the nucleus converting into a neutron while emitting a positron and a neutrino, requiring that the parent nucleus has an excess of protons and sufficient mass energy to allow this transformation. Beta decay, specifically beta-minus decay, occurs when a neutron transforms into a proton with the emission of an electron and an antineutrino, typically favored in neutron-rich nuclei due to energy differences governed by nuclear binding energy. Energy considerations in these transmutations depend on the mass-energy balance between the initial and final nuclei, with the process energetically allowed only when the final nucleus has a lower total mass-energy, ensuring the released kinetic energy distributes among decay particles.
Examples of Isotopes Undergoing Each Process
Isotopes such as Carbon-11 and Fluorine-18 commonly undergo positron emission, emitting a positron to transform into stable or different elements, primarily utilized in positron emission tomography (PET) imaging. Beta decay is frequently observed in isotopes like Carbon-14 and Tritium (Hydrogen-3), where a neutron transforms into a proton or vice versa by emitting a beta particle, playing a crucial role in radiometric dating and nuclear reactors. Both processes alter the atomic number and contribute significantly to nuclear medicine and environmental science via their respective isotopes.
Applications in Medicine and Industry
Positron emission tomography (PET) utilizes positron emission to produce detailed images of metabolic processes in the body, crucial for cancer diagnosis and neurological studies. Beta decay, involving the emission of electrons or positrons, is applied in industrial radiotracers to monitor material flow, detect leaks, and assess wear in pipelines. Both radioactive decay processes enable precise non-invasive techniques, enhancing medical diagnostics and industrial safety protocols.
Key Takeaways: Comparing Nuclear Decay Mechanisms
Positron emission involves the emission of a positron from a proton-rich nucleus, transforming a proton into a neutron and decreasing the atomic number by one, whereas beta decay features the emission of an electron (beta-minus) or positron (beta-plus) to stabilize the nucleus. Positron emission is a subset of beta-plus decay and occurs in isotopes with excess protons, while beta-minus decay occurs in neutron-rich isotopes, converting a neutron into a proton. Both decay types affect nuclear stability and are essential in medical imaging (PET scans) and radioactive tracing, with positron emission specifically utilized in positron emission tomography for cancer diagnostics.
Positron Emission Infographic
 libterm.com
libterm.com