Inorganic compounds are chemical substances that do not contain carbon-hydrogen bonds, distinguishing them from organic compounds. These substances include salts, metals, and minerals, which play crucial roles in various industrial and biological processes. Explore the full article to understand how inorganic materials impact your daily life and the environment.
Table of Comparison
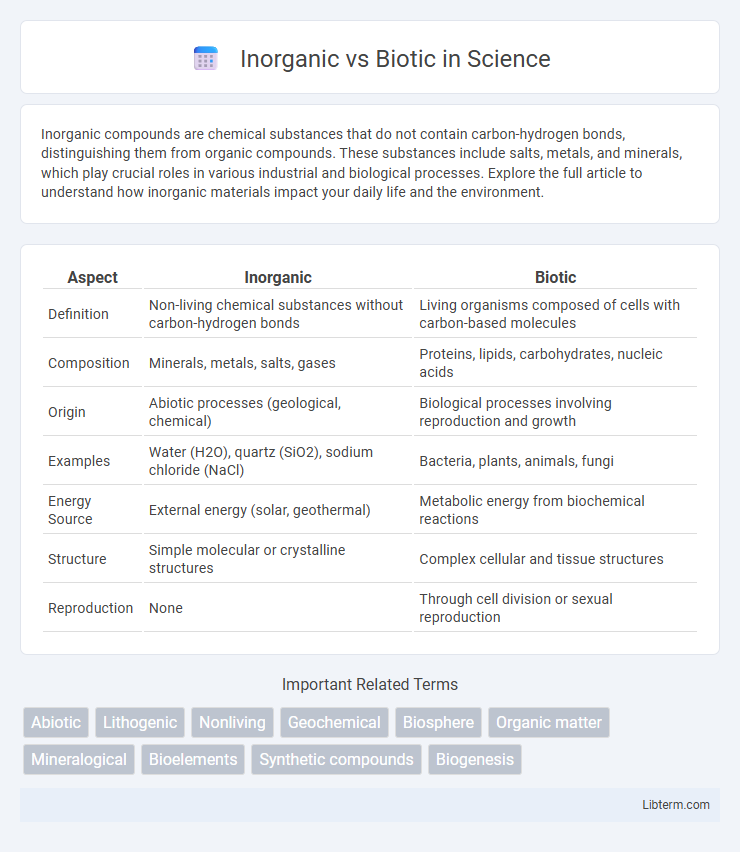
| Aspect | Inorganic | Biotic |
|---|---|---|
| Definition | Non-living chemical substances without carbon-hydrogen bonds | Living organisms composed of cells with carbon-based molecules |
| Composition | Minerals, metals, salts, gases | Proteins, lipids, carbohydrates, nucleic acids |
| Origin | Abiotic processes (geological, chemical) | Biological processes involving reproduction and growth |
| Examples | Water (H2O), quartz (SiO2), sodium chloride (NaCl) | Bacteria, plants, animals, fungi |
| Energy Source | External energy (solar, geothermal) | Metabolic energy from biochemical reactions |
| Structure | Simple molecular or crystalline structures | Complex cellular and tissue structures |
| Reproduction | None | Through cell division or sexual reproduction |
Defining Inorganic and Biotic
Inorganic substances encompass minerals, metals, and non-living chemicals that lack carbon-hydrogen bonds, forming the basis of geology and chemistry studies. Biotic components refer to living organisms, including plants, animals, bacteria, and fungi, fundamentally characterized by organic molecules and biological processes. Distinguishing inorganic from biotic hinges on the presence of life and organic compounds, crucial for ecological and environmental science classifications.
Key Characteristics of Inorganic Matter
Inorganic matter is characterized by the absence of carbon-hydrogen bonds and typically includes minerals, metals, and salts, which are non-living substances essential for various geological and chemical processes. These substances are generally stable, have definite chemical compositions, and lack the complex organic molecules found in biotic matter. Unlike biotic matter, inorganic materials do not decompose through biological processes and are fundamental in forming Earth's crust and influencing environmental conditions.
Essential Features of Biotic Components
Biotic components consist of living organisms such as plants, animals, fungi, and microorganisms that interact within ecosystems, playing crucial roles in processes like nutrient cycling, energy flow, and population dynamics. These components exhibit characteristics such as growth, reproduction, metabolism, and response to environmental stimuli, distinguishing them from inorganic, non-living substances. Essential features of biotic components include cellular structure, genetic material for heredity, and the ability to adapt to environmental changes, which sustain ecosystem functionality and biodiversity.
Origins and Formation: Inorganic vs Biotic
Inorganic substances originate from non-living chemical processes such as mineral crystallization and elemental reactions occurring in Earth's crust, often forming through geological phenomena like volcanic activity and sedimentation. Biotic materials derive from living organisms or organic matter, formed through biological processes like photosynthesis and decomposition, resulting in compounds such as carbohydrates, proteins, and lipids. The key distinction lies in inorganic compounds lacking carbon-hydrogen bonds, while biotic substances fundamentally involve organic carbon structures produced by or related to life.
Roles in Ecosystems: Comparative Analysis
Inorganic components such as minerals, water, and sunlight provide essential abiotic factors that regulate ecosystem processes like nutrient cycling and energy flow. Biotic elements, including plants, animals, and microbes, actively participate in food webs, decomposition, and habitat formation, driving biodiversity and ecological balance. The interaction between inorganic and biotic elements defines ecosystem structure and function, influencing productivity, resilience, and nutrient availability.
Chemical Composition and Structure
Inorganic compounds typically consist of simple molecules or ionic crystals formed by elements such as metals, nonmetals, and minerals, lacking carbon-hydrogen bonds. Biotic compounds are primarily organic, composed of complex carbon-based molecules like proteins, nucleic acids, lipids, and carbohydrates that exhibit diverse molecular architectures. Chemical structure in inorganic substances is often rigid and crystalline, while biotic molecules display dynamic, flexible structures crucial for biological function and interaction.
Interactions with the Environment
Inorganic substances interact with the environment primarily through physical and chemical processes such as mineral weathering, erosion, and nutrient cycling that influence soil composition and atmospheric gases. Biotic components, including plants, animals, and microorganisms, engage dynamically with their surroundings by modifying habitat structures, driving energy flow through food webs, and facilitating biogeochemical cycles like carbon and nitrogen fixation. These interactions between inorganic and biotic systems sustain ecosystem functions and regulate environmental conditions essential for life.
Significance in Earth's Cycles
Inorganic components such as minerals and water shape Earth's physical environment and drive processes like erosion, sedimentation, and the water cycle. Biotic elements, including plants, animals, and microbes, influence nutrient cycling, carbon sequestration, and energy flow within ecosystems. The interaction between inorganic and biotic factors is crucial for maintaining Earth's biogeochemical cycles and sustaining life.
Practical Applications and Examples
Inorganic compounds like salts, metals, and minerals are widely used in industrial manufacturing, water treatment, and electronics due to their stability and conductivity. Biotic substances, derived from living organisms such as proteins, enzymes, and biofuels, play crucial roles in pharmaceuticals, agriculture, and renewable energy. Practical applications of inorganic materials include fertilizers and catalysts, whereas biotic materials are essential in biodegradable plastics and medical therapies.
Conclusion: Understanding Their Differences
Inorganic substances consist of minerals, metals, and non-living chemical compounds, characterized by the absence of carbon-hydrogen bonds, whereas biotic components include living organisms and organic materials containing carbon-based molecules. Recognizing these fundamental differences is essential for fields such as environmental science, chemistry, and biology, as it influences the study of ecosystems, chemical reactions, and life processes. Distinguishing between inorganic and biotic elements aids in accurately analyzing natural phenomena and developing sustainable solutions.
Inorganic Infographic
 libterm.com
libterm.com