ELISA, or Enzyme-Linked Immunosorbent Assay, is a highly sensitive technique used to detect and quantify specific proteins, antibodies, or hormones in a sample. This method relies on antigen-antibody interactions and enzyme-mediated color changes to provide accurate and reliable results essential in diagnostics and research. Discover how ELISA can empower your analysis by exploring the detailed mechanisms and applications in the rest of this article.
Table of Comparison
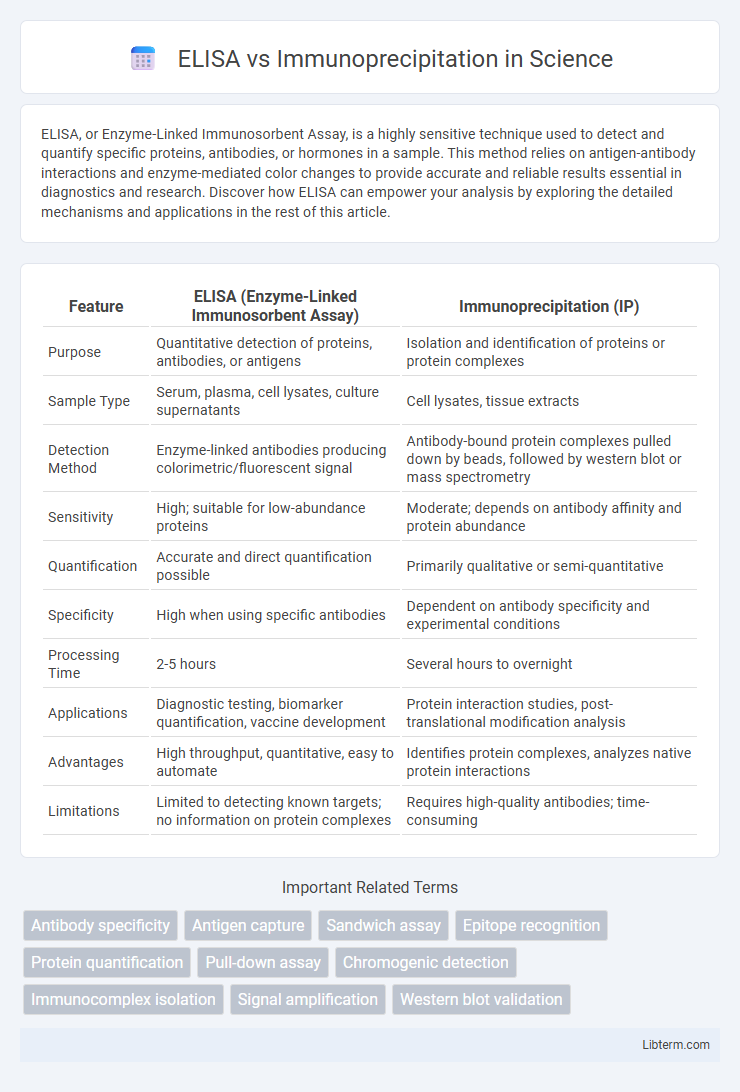
| Feature | ELISA (Enzyme-Linked Immunosorbent Assay) | Immunoprecipitation (IP) |
|---|---|---|
| Purpose | Quantitative detection of proteins, antibodies, or antigens | Isolation and identification of proteins or protein complexes |
| Sample Type | Serum, plasma, cell lysates, culture supernatants | Cell lysates, tissue extracts |
| Detection Method | Enzyme-linked antibodies producing colorimetric/fluorescent signal | Antibody-bound protein complexes pulled down by beads, followed by western blot or mass spectrometry |
| Sensitivity | High; suitable for low-abundance proteins | Moderate; depends on antibody affinity and protein abundance |
| Quantification | Accurate and direct quantification possible | Primarily qualitative or semi-quantitative |
| Specificity | High when using specific antibodies | Dependent on antibody specificity and experimental conditions |
| Processing Time | 2-5 hours | Several hours to overnight |
| Applications | Diagnostic testing, biomarker quantification, vaccine development | Protein interaction studies, post-translational modification analysis |
| Advantages | High throughput, quantitative, easy to automate | Identifies protein complexes, analyzes native protein interactions |
| Limitations | Limited to detecting known targets; no information on protein complexes | Requires high-quality antibodies; time-consuming |
Overview of ELISA and Immunoprecipitation
ELISA (Enzyme-Linked Immunosorbent Assay) is a highly sensitive technique used to detect and quantify specific proteins, antibodies, or antigens in a sample by exploiting antibody-antigen interactions combined with enzyme-mediated colorimetric detection. Immunoprecipitation (IP) isolates a particular protein or protein complex from a mixture by using an antibody specific to the target, enabling analysis of protein-protein interactions or post-translational modifications. While ELISA provides quantitative measurement suitable for high-throughput screening, immunoprecipitation offers qualitative insights into protein complexes and molecular associations.
Principle and Workflow Comparison
ELISA (Enzyme-Linked Immunosorbent Assay) relies on antigen-antibody binding detected through enzyme-mediated colorimetric changes, enabling quantitative measurement of target proteins in samples. Immunoprecipitation (IP) involves antibody-based capture of specific proteins from complex mixtures, followed by their isolation using protein A/G beads and analysis typically via Western blot or mass spectrometry. ELISA offers high-throughput quantification with a structured, plate-based workflow, while immunoprecipitation provides detailed protein interaction and complex analysis through selective immunocapture and subsequent detection.
Applications in Research and Diagnostics
ELISA is widely used in research and diagnostics for quantifying specific proteins, antibodies, and hormones in complex samples, enabling high-throughput screening and disease biomarker detection. Immunoprecipitation excels in studying protein-protein interactions, post-translational modifications, and isolating target antigens from cell lysates for functional analysis. Both techniques are essential tools: ELISA provides sensitive, quantitative results ideal for diagnostic assays, while immunoprecipitation offers molecular insights into protein complexes crucial for mechanistic research.
Sensitivity and Specificity Analysis
ELISA demonstrates high sensitivity by detecting low concentrations of antigens through enzyme-linked antibodies, making it suitable for quantitative analysis in various samples. Immunoprecipitation offers superior specificity by isolating antigen-antibody complexes, enabling the identification of target proteins within complex mixtures. Sensitivity in immunoprecipitation is generally lower than ELISA due to reliance on antibody affinity and precipitation efficiency, while ELISA provides a broader dynamic range for detecting analytes.
Sample Types and Preparation
ELISA is commonly used with serum, plasma, cell culture supernatants, and other liquid samples, requiring minimal preparation such as dilution or centrifugation to remove particulates. Immunoprecipitation demands more complex sample preparation, often involving cell or tissue lysates solubilized with detergents to maintain protein-protein interactions while preserving antigenicity. Sample type selection in immunoprecipitation typically depends on the need to study native protein complexes, whereas ELISA targets quantification of specific antigens in relatively clear biological fluids.
Quantitative vs Qualitative Output
ELISA provides quantitative data by measuring the concentration of specific antigens or antibodies through colorimetric changes, enabling precise quantification of target molecules. Immunoprecipitation offers qualitative insights by isolating and identifying protein interactions or complexes without directly measuring their amounts. The quantitative strength of ELISA makes it ideal for biomarker analysis, while immunoprecipitation excels in studying protein function and binding partners.
Throughput and Scalability
ELISA offers high throughput capabilities, enabling the simultaneous analysis of hundreds of samples with automated plate readers, making it ideal for large-scale studies and routine diagnostics. Immunoprecipitation, by contrast, has lower throughput due to its labor-intensive protocol and reliance on individual sample processing, limiting scalability for extensive sample sets. While ELISA scales efficiently in multi-well formats, immunoprecipitation is better suited for detailed protein interaction studies where throughput is less critical.
Time, Cost, and Resource Considerations
ELISA offers faster results, often within a few hours, making it suitable for high-throughput screening with lower per-sample costs due to minimal reagent use and automation potential. Immunoprecipitation involves longer processing times, typically several hours to overnight, necessitating more specialized equipment and skilled personnel, which increases overall expenses. Resource requirements for ELISA include microplate readers and standard laboratory supplies, whereas immunoprecipitation demands antibodies, beads, centrifuges, and often subsequent analysis like Western blotting.
Common Challenges and Limitations
ELISA and Immunoprecipitation both face challenges such as antibody specificity and cross-reactivity, which can compromise assay accuracy. Sensitivity limitations often affect the detection of low-abundance proteins, impacting quantitative results. Sample complexity and availability can further restrict assay efficiency and reproducibility in both techniques.
Selecting the Right Assay for Your Study
Choosing between ELISA and immunoprecipitation depends on the study's specific goals, such as quantifying protein concentration or analyzing protein interactions. ELISA offers high sensitivity and quantification of target antigens across multiple samples, making it ideal for large-scale screening and diagnostic purposes. Immunoprecipitation excels in isolating protein complexes and investigating protein-protein interactions, providing detailed insights into molecular mechanisms within cellular contexts.
ELISA Infographic
 libterm.com
libterm.com