Flow cytometry is a powerful technique used to analyze the physical and chemical characteristics of cells or particles suspended in a fluid stream. It enables rapid multiparametric analysis, making it essential for immunophenotyping, cell sorting, and biomarker detection in clinical and research settings. Discover how flow cytometry can enhance your cell analysis by exploring the detailed methods and applications in the rest of this article.
Table of Comparison
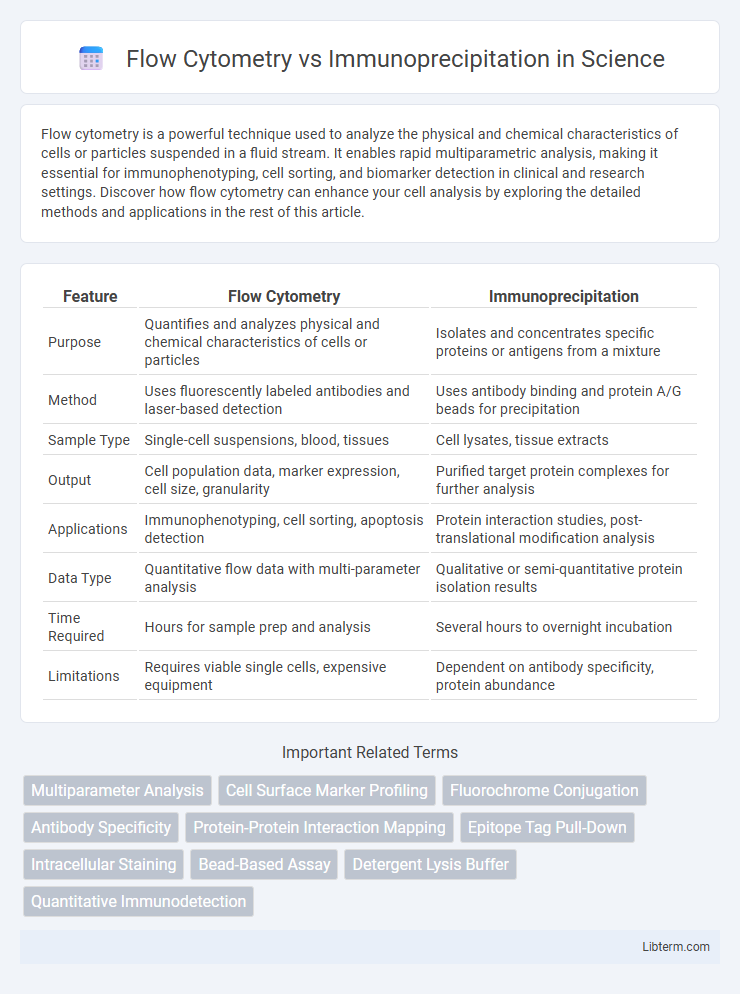
| Feature | Flow Cytometry | Immunoprecipitation |
|---|---|---|
| Purpose | Quantifies and analyzes physical and chemical characteristics of cells or particles | Isolates and concentrates specific proteins or antigens from a mixture |
| Method | Uses fluorescently labeled antibodies and laser-based detection | Uses antibody binding and protein A/G beads for precipitation |
| Sample Type | Single-cell suspensions, blood, tissues | Cell lysates, tissue extracts |
| Output | Cell population data, marker expression, cell size, granularity | Purified target protein complexes for further analysis |
| Applications | Immunophenotyping, cell sorting, apoptosis detection | Protein interaction studies, post-translational modification analysis |
| Data Type | Quantitative flow data with multi-parameter analysis | Qualitative or semi-quantitative protein isolation results |
| Time Required | Hours for sample prep and analysis | Several hours to overnight incubation |
| Limitations | Requires viable single cells, expensive equipment | Dependent on antibody specificity, protein abundance |
Introduction to Flow Cytometry and Immunoprecipitation
Flow cytometry is a laser-based biophysical technology used to analyze the physical and chemical characteristics of cells or particles suspended in a fluid stream, enabling rapid multiparametric analysis of thousands of cells per second. Immunoprecipitation is a biochemical technique that isolates a specific antigen from a mixture by using an antibody that specifically binds to that antigen, allowing for the study of protein-protein interactions and identification. Both methods provide critical insights into cellular function and protein dynamics but differ fundamentally in their approach--flow cytometry offers quantitative cell analysis, while immunoprecipitation focuses on molecular isolation and interaction.
Principles and Mechanisms of Flow Cytometry
Flow cytometry utilizes the principle of hydrodynamic focusing to align cells in a fluid stream, allowing laser-based detection of scattered light and fluorescence signals as each cell passes individually through the laser beam. It measures cellular properties such as size, granularity, and expression of surface or intracellular markers by tagging cells with fluorescently labeled antibodies specific to target antigens. Unlike immunoprecipitation, which isolates protein complexes using antibody-antigen binding and centrifugation, flow cytometry provides rapid multiparametric analysis and quantification of thousands of cells in real-time.
Principles and Mechanisms of Immunoprecipitation
Immunoprecipitation operates on the principle of using specific antibodies to selectively bind and isolate target antigens from complex protein mixtures, forming antibody-antigen complexes that can be precipitated by Protein A or G beads. This technique relies on the high affinity and specificity of antibodies to capture proteins, facilitating analysis of protein interactions, post-translational modifications, and expression levels. Unlike flow cytometry, which analyzes individual cells in suspension based on fluorescence-labeled markers, immunoprecipitation enables the extraction and enrichment of particular proteins for downstream applications such as Western blotting or mass spectrometry.
Key Differences Between Flow Cytometry and Immunoprecipitation
Flow cytometry quantifies and analyzes physical and chemical characteristics of cells or particles in a fluid stream, offering rapid multiparametric data on cell size, granularity, and marker expression using fluorescently labeled antibodies. Immunoprecipitation isolates and enriches specific proteins or protein complexes from cell lysates through antigen-antibody binding, enabling downstream analysis such as Western blot or mass spectrometry. Unlike immunoprecipitation's focus on protein purification and interaction studies, flow cytometry excels in high-throughput single-cell analysis and phenotyping based on surface and intracellular markers.
Applications of Flow Cytometry in Biomedical Research
Flow cytometry enables rapid, multiparametric analysis of individual cells, making it invaluable for immunophenotyping, cell cycle analysis, and apoptosis detection in biomedical research. This technique quantifies surface and intracellular biomarkers, facilitating detailed studies of cellular responses and disease states such as cancer, autoimmune disorders, and infectious diseases. Compared to immunoprecipitation, which isolates specific proteins or protein complexes, flow cytometry provides high-throughput, single-cell resolution data essential for diagnostics and therapeutic monitoring.
Applications of Immunoprecipitation in Molecular Biology
Immunoprecipitation is a powerful technique used in molecular biology to isolate and analyze specific proteins or protein complexes from complex mixtures, enabling the study of protein-protein interactions, post-translational modifications, and antigen-antibody binding specificity. This method plays a crucial role in identifying signaling pathways, detecting protein expression levels, and characterizing cellular mechanisms underlying diseases such as cancer and autoimmune disorders. Compared to flow cytometry, which is primarily used for cell sorting and phenotypic analysis, immunoprecipitation provides direct insights into molecular interactions at the protein level.
Advantages and Limitations of Flow Cytometry
Flow cytometry offers rapid, quantitative analysis of thousands of cells per second, providing detailed multiparametric data on cell size, granularity, and protein expression with high sensitivity. Its limitations include the need for specialized equipment, complex data analysis, and potential false positives due to non-specific antibody binding. Compared to immunoprecipitation, flow cytometry excels in high-throughput single-cell analysis but is less effective for studying protein-protein interactions or purifying specific proteins from complex mixtures.
Advantages and Limitations of Immunoprecipitation
Immunoprecipitation offers high specificity in isolating target proteins or protein complexes from complex biological samples, enabling detailed analysis of protein-protein interactions and post-translational modifications. However, its limitations include a dependency on the availability and quality of specific antibodies, potential co-precipitation of non-target proteins causing background noise, and a time-intensive protocol that may not be suitable for high-throughput analysis. Compared to flow cytometry, immunoprecipitation provides molecular-level insights but lacks the ability to analyze large cell populations rapidly or provide information on cellular heterogeneity.
Choosing the Right Technique: Factors to Consider
Choosing between flow cytometry and immunoprecipitation depends on the experimental goal, such as whether quantifying cell populations or analyzing protein-protein interactions is the primary focus. Flow cytometry excels in multiparametric single-cell analysis and rapid quantification of surface or intracellular markers, while immunoprecipitation is ideal for isolating specific proteins or complexes from lysates for downstream applications like western blotting or mass spectrometry. Consider sample type, required throughput, sensitivity, and data complexity to determine the most appropriate technique for your research needs.
Future Perspectives and Emerging Trends in Protein Analysis
Flow cytometry is advancing with high-dimensional multiplexing capabilities and integration of machine learning algorithms, enabling detailed single-cell protein profiling and dynamic cellular interaction studies. Immunoprecipitation techniques are evolving through enhanced antibody specificity and coupling with mass spectrometry for deeper protein complex characterization and post-translational modification analysis. Emerging trends emphasize combining these methods with novel biosensors and microfluidics to improve sensitivity, throughput, and real-time monitoring in proteomics research.
Flow Cytometry Infographic
 libterm.com
libterm.com