Endothermic processes absorb heat from their surroundings, resulting in a temperature drop in the environment. These reactions are essential in various industrial applications and biological systems where energy input is required to drive change. Explore the rest of the article to understand how endothermic reactions impact your daily life and the world around you.
Table of Comparison
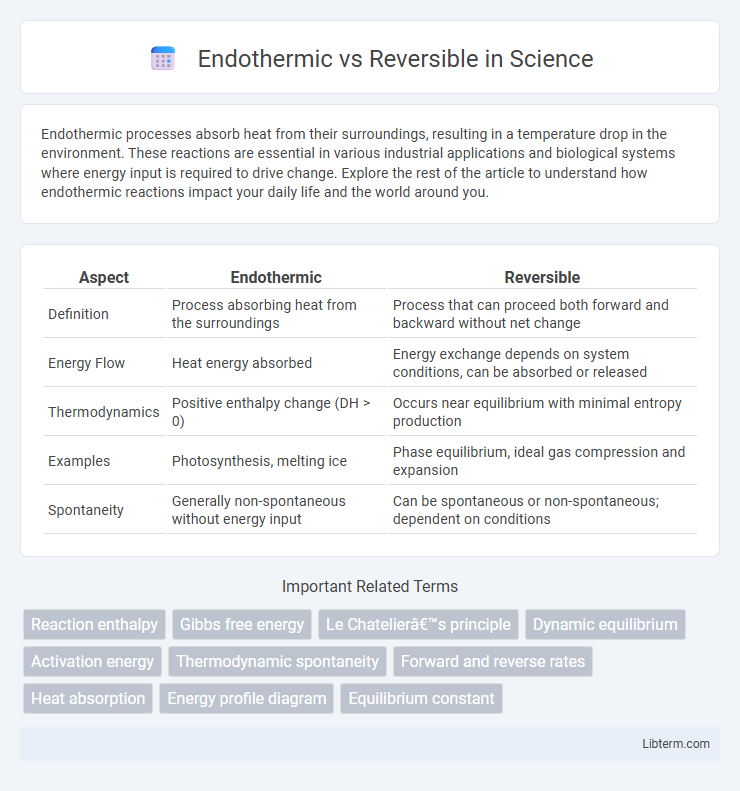
| Aspect | Endothermic | Reversible |
|---|---|---|
| Definition | Process absorbing heat from the surroundings | Process that can proceed both forward and backward without net change |
| Energy Flow | Heat energy absorbed | Energy exchange depends on system conditions, can be absorbed or released |
| Thermodynamics | Positive enthalpy change (DH > 0) | Occurs near equilibrium with minimal entropy production |
| Examples | Photosynthesis, melting ice | Phase equilibrium, ideal gas compression and expansion |
| Spontaneity | Generally non-spontaneous without energy input | Can be spontaneous or non-spontaneous; dependent on conditions |
Introduction to Endothermic and Reversible Processes
Endothermic processes absorb heat energy, causing the system's temperature to decrease while the surroundings warm up. Reversible processes occur in such a way that the system and surroundings can be returned to their initial states without any net change in entropy. Understanding the differences between endothermic and reversible processes is crucial for analyzing thermodynamic systems and energy transfer efficiency.
Defining Endothermic Reactions
Endothermic reactions absorb energy from their surroundings, typically in the form of heat, resulting in a temperature decrease in the environment. These reactions require continuous energy input to proceed, distinguishing them from exothermic processes that release energy. Understanding endothermic reactions is crucial for analyzing reversible reactions, where the direction depends on energy changes and equilibrium conditions.
Understanding Reversible Reactions
Reversible reactions occur when the reactants convert to products and the products simultaneously convert back to reactants, achieving a dynamic equilibrium where the forward and reverse reaction rates are equal. These reactions often involve energy changes, with endothermic processes absorbing heat in one direction and releasing it when reversed. Understanding reversible reactions is crucial for controlling chemical equilibria in industrial applications, such as ammonia synthesis in the Haber process.
Key Differences Between Endothermic and Reversible Reactions
Endothermic reactions absorb heat from their surroundings, resulting in a temperature decrease, whereas reversible reactions can proceed in both forward and backward directions, allowing the system to reach dynamic equilibrium. Endothermic processes are defined by a positive change in enthalpy (DH > 0), while reversible reactions are characterized by the ability to restore the initial state without net energy loss. The key difference lies in energy transfer for endothermic reactions versus the directional reversibility and equilibrium state in reversible reactions.
Energy Changes in Endothermic Reactions
Endothermic reactions absorb energy from their surroundings, resulting in a positive enthalpy change (DH > 0). The energy absorbed is used to break chemical bonds in the reactants, leading to an increase in the system's internal energy. In reversible reactions, the direction depends on energy changes and equilibrium conditions, where endothermic processes can shift toward product formation if heat is supplied.
Thermodynamics of Reversible Reactions
Reversible reactions in thermodynamics occur when reactants and products are in dynamic equilibrium, allowing the reaction to proceed in both forward and backward directions without net energy loss. These reactions are characterized by minimal entropy production, enabling the system to maintain maximum thermodynamic efficiency. In contrast, endothermic reactions absorb heat from the surroundings, affecting the enthalpy change but not necessarily dictating reversibility in the thermodynamic sense.
Examples of Endothermic Reactions
Photosynthesis is a prime example of an endothermic reaction, where plants absorb sunlight to convert carbon dioxide and water into glucose and oxygen. Another common endothermic process is the melting of ice, which requires heat energy to change from solid to liquid. The decomposition of calcium carbonate into calcium oxide and carbon dioxide during thermal decomposition also demonstrates an endothermic reaction, absorbing heat to proceed.
Examples of Reversible Reactions
Reversible reactions, such as the synthesis of ammonia in the Haber process (N2 + 3H2 = 2NH3) and the esterification of carboxylic acids with alcohols (RCOOH + R'OH = RCOOR' + H2O), involve processes where reactants and products coexist in dynamic equilibrium. The endothermic decomposition of calcium carbonate (CaCO3 = CaO + CO2) demonstrates that reversible reactions can absorb heat, shifting equilibrium based on temperature changes. These examples highlight the fundamental characteristic of reversible reactions to respond to environmental variables, enabling control over chemical yields.
Real-World Applications of Endothermic and Reversible Processes
Endothermic processes, such as photosynthesis and industrial ammonia synthesis, absorb heat to drive essential chemical reactions, enabling energy storage and production. Reversible processes, exemplified by phase changes like melting and vaporization, allow systems to return to their original state without net energy loss, crucial in thermal energy management and material recycling. These mechanisms underpin advancements in sustainable energy technologies, chemical manufacturing, and efficient heat exchange systems.
Summary: Choosing Between Endothermic and Reversible Reactions
Endothermic reactions absorb heat, requiring continuous energy input to proceed, while reversible reactions can move forward or backward depending on conditions like temperature and pressure. Selecting between endothermic and reversible processes depends on the desired reaction control, energy efficiency, and equilibrium maintenance. Understanding thermodynamic parameters such as enthalpy changes and equilibrium constants is essential for optimizing industrial chemical production and laboratory synthesis.
Endothermic Infographic
 libterm.com
libterm.com