Diastereomeric compounds are stereoisomers that are not mirror images of each other, differing in the configuration at one or more stereocenters. They exhibit distinct physical and chemical properties, making their separation and identification crucial in synthetic chemistry and pharmaceuticals. Explore the rest of the article to understand how diastereomeric relationships impact molecular behavior and practical applications.
Table of Comparison
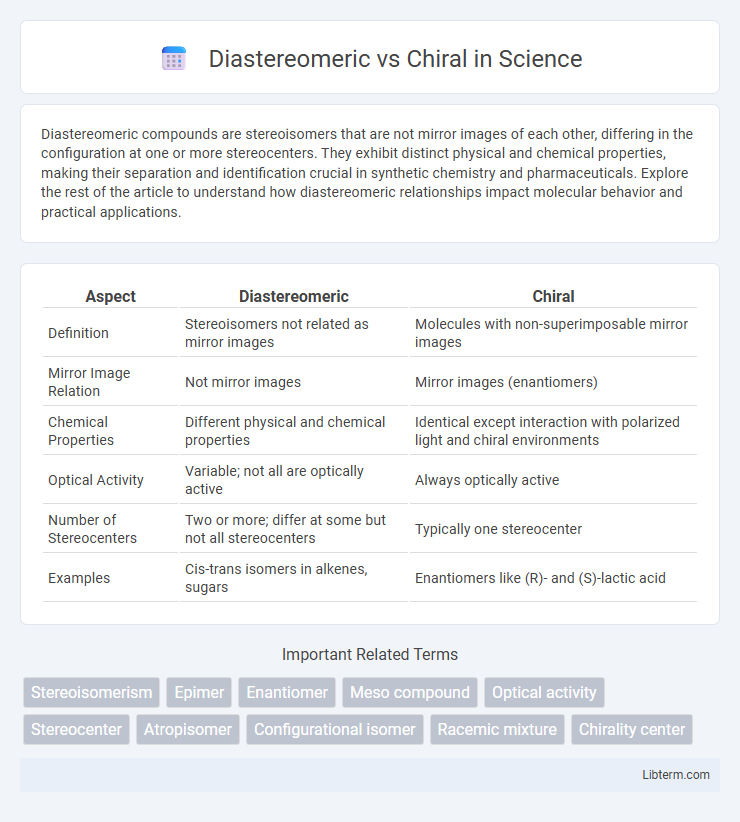
| Aspect | Diastereomeric | Chiral |
|---|---|---|
| Definition | Stereoisomers not related as mirror images | Molecules with non-superimposable mirror images |
| Mirror Image Relation | Not mirror images | Mirror images (enantiomers) |
| Chemical Properties | Different physical and chemical properties | Identical except interaction with polarized light and chiral environments |
| Optical Activity | Variable; not all are optically active | Always optically active |
| Number of Stereocenters | Two or more; differ at some but not all stereocenters | Typically one stereocenter |
| Examples | Cis-trans isomers in alkenes, sugars | Enantiomers like (R)- and (S)-lactic acid |
Understanding Diastereomers: Key Definitions
Diastereomers are stereoisomers that are not mirror images of each other and differ in the configuration of one or more, but not all, chiral centers within a molecule. Unlike enantiomers, which are non-superimposable mirror images, diastereomers have distinct physical and chemical properties, such as melting points and reactivities. Understanding the difference between diastereomers and chiral enantiomers is crucial for predicting stereochemical outcomes in organic synthesis and drug design.
What Does "Chiral" Mean in Chemistry?
Chiral in chemistry refers to a molecule that cannot be superimposed on its mirror image, often due to the presence of an asymmetric carbon atom bonded to four different groups. This property leads to the existence of enantiomers, which are pairs of chiral molecules with identical physical properties except for their interaction with plane-polarized light and chiral environments. Diastereomers, by contrast, are stereoisomers that are not mirror images, differing in physical and chemical properties.
Structural Differences: Diastereomers vs Chiral Compounds
Diastereomers differ from chiral compounds primarily in their stereochemical relationships; diastereomers are stereoisomers that are not mirror images and typically have multiple stereocenters with differing configurations at one or more sites. Chiral compounds possess a single stereocenter or an arrangement that lacks an internal plane of symmetry, leading to non-superimposable mirror images called enantiomers. Structural differences between diastereomers and chiral molecules affect their physical properties, optical activities, and biological interactions distinctly.
Stereochemistry Fundamentals: Chiral Centers and Diastereomers
Chiral molecules contain at least one chiral center, typically a carbon atom bonded to four different substituents, resulting in non-superimposable mirror images called enantiomers. Diastereomers are stereoisomers that are not mirror images and arise when molecules have multiple chiral centers with differing configurations at one or more of these centers. Understanding the spatial arrangement of atoms around chiral centers is fundamental in stereochemistry for distinguishing between diastereomers and chiral molecules.
Optical Activity: How Diastereomers and Chiral Molecules Differ
Chiral molecules exhibit optical activity by rotating plane-polarized light due to their non-superimposable mirror images called enantiomers. Diastereomers, in contrast, are stereoisomers that are not mirror images and may have different physical properties but do not necessarily show optical activity. Optical rotation in chiral compounds is a key characteristic for identifying enantiomers, whereas diastereomers typically differ in melting points, boiling points, and other physical constants without consistent optical behavior.
Methods of Identifying Diastereomers and Chiral Molecules
Diastereomers can be identified through physical property comparisons such as melting points, boiling points, and solubility, as they exhibit distinct differences unlike enantiomers. Chiral molecules are often identified using polarimetry to measure optical rotation or chiral chromatography techniques like HPLC with chiral stationary phases. Nuclear Magnetic Resonance (NMR) spectroscopy with chiral shift reagents also aids in distinguishing between diastereomers and chiral molecules by analyzing their unique spectral patterns.
Real-world Examples: Diastereomeric and Chiral Compounds
Diastereomers, such as glucose and galactose, differ in the spatial arrangement of atoms at one or more stereocenters but are not mirror images, leading to distinct physical and chemical properties crucial in pharmaceuticals. Chiral compounds like L-amino acids exist as non-superimposable mirror images, playing vital roles in biological systems where only one enantiomer is bioactive. The distinction between diastereomers and chiral molecules influences drug efficacy and safety, exemplified by thalidomide's enantiomers having drastically different biological effects.
Importance in Drug Development: Chiral vs Diastereomeric Compounds
Chiral compounds play a crucial role in drug development due to their ability to exist as enantiomers with distinct biological activities, affecting drug efficacy and safety. Diastereomeric compounds, having multiple stereocenters, exhibit different physical and chemical properties, making their separation and characterization vital for optimizing pharmacological profiles. Understanding the differences between chiral and diastereomeric compounds enables precise control over therapeutic effects and reduces adverse reactions in pharmaceutical design.
Separation Techniques: Resolving Diastereomers and Chiral Molecules
Diastereomers can often be separated using conventional chromatographic methods such as silica gel column chromatography or preparative thin-layer chromatography due to their differing physical properties like polarity and solubility. Chiral molecules require specialized techniques like chiral stationary phase high-performance liquid chromatography (HPLC) or supercritical fluid chromatography (SFC) to achieve enantiomeric resolution, exploiting interactions with chiral selectors. Crystallization methods including diastereomeric salt formation and chiral resolution via enzymatic treatment also effectively separate chiral molecules by creating distinct physical entities.
Summary Table: Diastereomeric vs Chiral Comparison
Diastereomeric compounds are stereoisomers that are not mirror images of each other and differ in multiple stereocenters, whereas chiral molecules possess at least one stereocenter and lack an internal plane of symmetry, resulting in non-superimposable mirror images called enantiomers. The summary table comparing diastereomeric and chiral compounds highlights key differences such as molecular symmetry, optical activity, number of stereocenters involved, and physical/chemical property variations. Diastereomers exhibit distinct physical and chemical properties unlike enantiomers, which share most properties except for the direction of optical rotation and interactions with other chiral substances.
Diastereomeric Infographic
 libterm.com
libterm.com