Lewis acids are chemical species that can accept an electron pair, making them essential in numerous catalytic and synthetic processes. Their ability to interact with electron-rich molecules influences reaction mechanisms and product formation in organic and inorganic chemistry. Discover how understanding Lewis acids can enhance your grasp of chemical reactivity by reading the rest of this article.
Table of Comparison
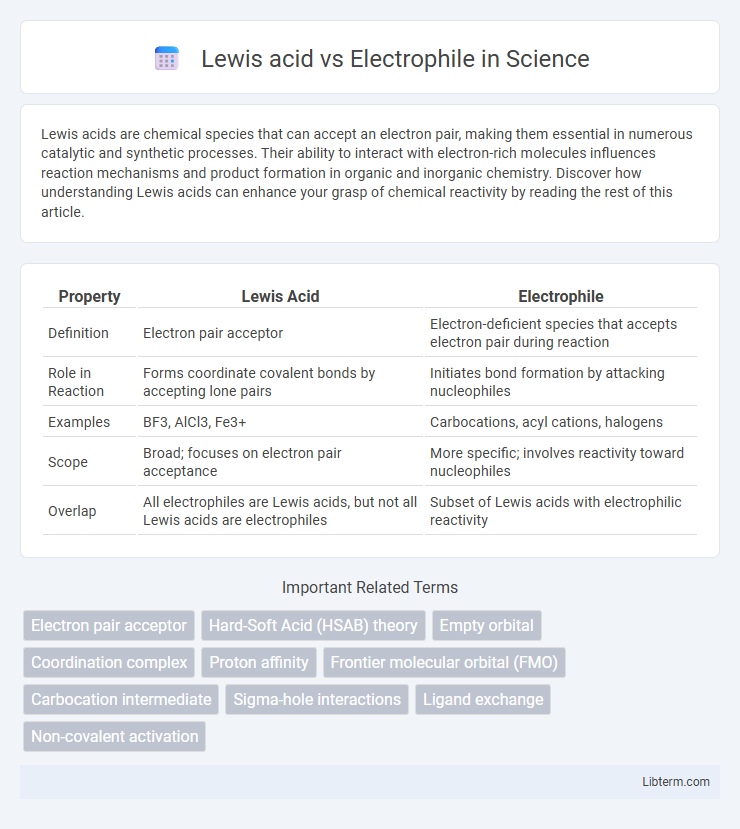
| Property | Lewis Acid | Electrophile |
|---|---|---|
| Definition | Electron pair acceptor | Electron-deficient species that accepts electron pair during reaction |
| Role in Reaction | Forms coordinate covalent bonds by accepting lone pairs | Initiates bond formation by attacking nucleophiles |
| Examples | BF3, AlCl3, Fe3+ | Carbocations, acyl cations, halogens |
| Scope | Broad; focuses on electron pair acceptance | More specific; involves reactivity toward nucleophiles |
| Overlap | All electrophiles are Lewis acids, but not all Lewis acids are electrophiles | Subset of Lewis acids with electrophilic reactivity |
Introduction to Lewis Acids and Electrophiles
Lewis acids are chemical species that accept an electron pair, playing a crucial role in chemical reactions by interacting with Lewis bases. Electrophiles are atoms or molecules that seek electrons and react with nucleophiles during bond formation, often overlapping with Lewis acids in their electron-accepting behavior. Understanding the distinction between Lewis acids and electrophiles involves recognizing that all Lewis acids are electrophiles, but not all electrophiles qualify as Lewis acids, especially when considering their specific reaction mechanisms and contexts.
Fundamental Definitions: Lewis Acid vs Electrophile
A Lewis acid is defined as a chemical species that can accept an electron pair to form a covalent bond, whereas an electrophile refers to an atom or molecule that seeks electrons and is attracted to electron-rich sites in a chemical reaction. Both concepts involve electron acceptance, but Lewis acids are specifically characterized by their ability to accept a lone pair from a Lewis base, while electrophiles participate more broadly in reactions by attacking nucleophilic centers. Understanding the distinction is crucial in organic chemistry mechanisms, where Lewis acids often act as catalysts and electrophiles as reactive intermediates.
Theoretical Background: Electron Pair Acceptance
Lewis acids are defined by their ability to accept an electron pair, making them electron pair acceptors in chemical reactions. Electrophiles are species that seek out electron-rich areas to accept electrons, often overlapping with Lewis acid behavior but generally focused on reaction sites rather than formal electron pair acceptance. The theoretical distinction centers on electron pair acceptance as a fundamental property of Lewis acids, while electrophiles encompass a broader range of electron-seeking entities in organic and inorganic chemistry.
Key Differences Between Lewis Acids and Electrophiles
Lewis acids are defined by their ability to accept an electron pair, often characterized by empty orbitals or positive charges, while electrophiles seek electron-rich regions to form new bonds, frequently involving partial positive charges. Lewis acids operate primarily through coordinate covalent bond formation by directly accepting electron pairs, whereas electrophiles engage in broader reactions such as nucleophilic attack and may not always form classic coordinate bonds. The key difference lies in the scope: all Lewis acids are electrophiles due to their electron pair acceptance but not all electrophiles qualify as Lewis acids, as some electrophiles react through mechanisms beyond simple electron pair acceptance.
Structural Characteristics and Examples
Lewis acids are electron pair acceptors characterized by vacant orbitals or positive charges, exemplified by boron trifluoride (BF3) and aluminum chloride (AlCl3), which facilitate coordination with electron-rich species. Electrophiles are species that seek electrons to form covalent bonds, often containing positive charges or partial positive centers, such as carbocations and halogen molecules like Br2, enabling reactions with nucleophiles. Structurally, Lewis acids often possess empty p-orbitals or metal centers, while electrophiles may have positively polarized atoms or electron-deficient carbons, influencing their reactivity and interaction in organic and inorganic reactions.
Mechanisms of Action in Chemical Reactions
Lewis acids act as electron pair acceptors, stabilizing negative charge buildup by coordinating with lone pairs during chemical reactions, often facilitating bond formation or cleavage. Electrophiles seek electron-rich sites to form new bonds by accepting electrons, driving nucleophilic attack mechanisms through partial or full positive charges. The distinction lies in Lewis acids emphasizing orbital interactions with electron pairs, while electrophiles function primarily through charge-driven attraction to nucleophiles.
Overlapping Concepts: When Lewis Acids Act as Electrophiles
Lewis acids function as electron pair acceptors and often act as electrophiles by seeking electron-rich sites in molecules during chemical reactions. Both Lewis acids and electrophiles overlap in their reactivity because they accept electrons, but Lewis acids specifically require empty orbitals to accept lone pairs, whereas electrophiles are broader and include species that accept electrons through different mechanisms. This overlap is critical in catalysis and organic synthesis, where Lewis acids activate electrophilic centers to facilitate bond formation.
Unique Roles in Organic and Inorganic Chemistry
Lewis acids act as electron pair acceptors primarily in inorganic chemistry, facilitating complex formation and catalyzing reactions by stabilizing negative charges. Electrophiles, characterized by electron deficiency, target nucleophilic sites in organic molecules to form new covalent bonds, playing a crucial role in substitution and addition reactions. The unique role of Lewis acids lies in their ability to coordinate with lone pairs, while electrophiles drive bond formation through direct attack on electron-rich centers.
Practical Applications in Synthesis and Catalysis
Lewis acids function as electron pair acceptors and play a crucial role in catalyzing organic reactions such as Friedel-Crafts alkylation and Diels-Alder cycloadditions by activating electrophiles or stabilizing reaction intermediates. Electrophiles, which seek electrons, are central to initiating nucleophilic attack in substitution and addition reactions, widely used in the synthesis of pharmaceuticals and agrochemicals. Both Lewis acids and electrophiles enable selective bond formation and transformation, enhancing reaction efficiency and product yield in synthetic chemistry and industrial catalysis.
Conclusion: Importance in Chemical Understanding
Lewis acids and electrophiles play distinct roles in chemical reactions; Lewis acids accept electron pairs from donors, while electrophiles seek electrons to form bonds but may not always accept lone pairs directly. Understanding their differences enhances the prediction of reaction mechanisms and the design of catalysts in organic and inorganic chemistry. This distinction is crucial for developing targeted synthetic strategies and improving reaction efficiency in research and industrial applications.
Lewis acid Infographic
 libterm.com
libterm.com