Electron capture is a nuclear decay process where an inner orbital electron is absorbed by the nucleus, converting a proton into a neutron and emitting a neutrino. This process alters the atomic number of the element without changing its mass number, leading to the formation of a new element. Discover how electron capture impacts nuclear stability and applications by reading the rest of the article.
Table of Comparison
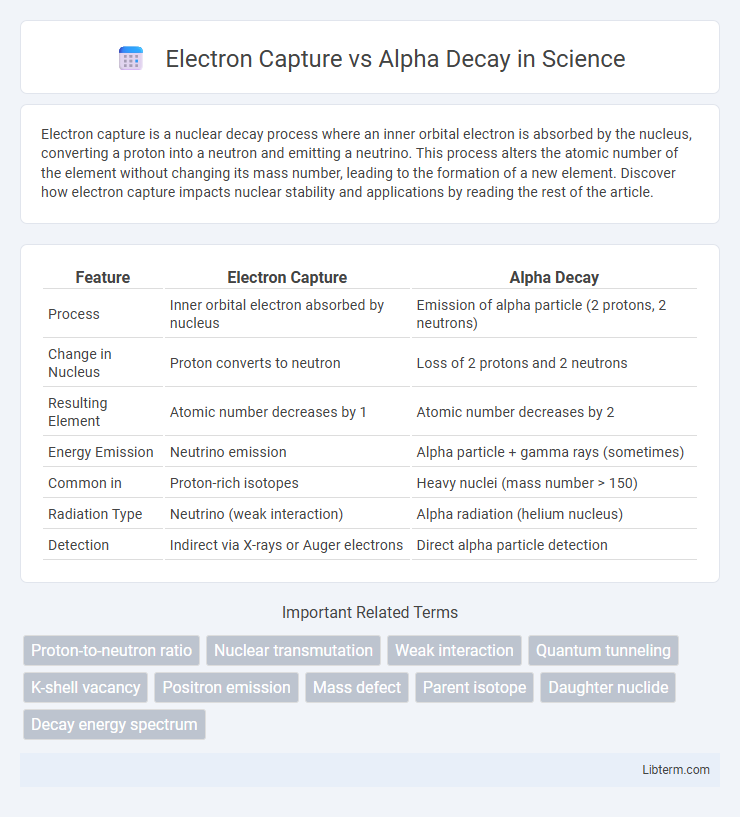
| Feature | Electron Capture | Alpha Decay |
|---|---|---|
| Process | Inner orbital electron absorbed by nucleus | Emission of alpha particle (2 protons, 2 neutrons) |
| Change in Nucleus | Proton converts to neutron | Loss of 2 protons and 2 neutrons |
| Resulting Element | Atomic number decreases by 1 | Atomic number decreases by 2 |
| Energy Emission | Neutrino emission | Alpha particle + gamma rays (sometimes) |
| Common in | Proton-rich isotopes | Heavy nuclei (mass number > 150) |
| Radiation Type | Neutrino (weak interaction) | Alpha radiation (helium nucleus) |
| Detection | Indirect via X-rays or Auger electrons | Direct alpha particle detection |
Introduction to Nuclear Decay Processes
Electron capture and alpha decay are two fundamental nuclear decay processes that alter the composition of an atomic nucleus to achieve greater stability. Electron capture involves the nucleus absorbing an inner orbital electron, transforming a proton into a neutron and decreasing the atomic number by one without changing the mass number. Alpha decay ejects an alpha particle, consisting of two protons and two neutrons, from the nucleus, reducing both the atomic number by two and the mass number by four, commonly observed in heavy elements like uranium and thorium.
What is Electron Capture?
Electron capture is a nuclear process in which an inner atomic electron is absorbed by the nucleus, converting a proton into a neutron and emitting a neutrino. This transformation decreases the atomic number by one while keeping the mass number constant. Electron capture commonly occurs in proton-rich isotopes and plays a vital role in nuclear stability and radioactive decay pathways.
Understanding Alpha Decay
Alpha decay involves the emission of an alpha particle, consisting of two protons and two neutrons, from a radioactive nucleus, leading to the formation of a new element with a decreased atomic number by two and mass number by four. This process is common in heavy elements like uranium and thorium, where the nucleus becomes unstable due to an excess of protons and neutrons. Understanding alpha decay is crucial for applications in nuclear physics, radiometric dating, and the production of medical isotopes.
Mechanisms: How Electron Capture Occurs
Electron capture occurs when an inner orbital electron is captured by the nucleus, combining with a proton to form a neutron and emitting a neutrino in the process. This mechanism reduces the atomic number by one while keeping the mass number constant, typically observed in proton-rich isotopes. The process competes with alpha decay in certain nuclei, influencing nuclear stability and transformation pathways.
Mechanisms: How Alpha Decay Occurs
Alpha decay occurs when an unstable nucleus emits an alpha particle consisting of two protons and two neutrons, reducing its atomic number by two and mass number by four. This process happens because the nuclear binding energy is minimized by releasing the alpha particle, allowing the nucleus to reach a more stable configuration. Quantum tunneling enables the alpha particle to overcome the Coulomb barrier despite insufficient classical energy.
Differences in Emitted Particles
Electron capture involves the nucleus absorbing an inner orbital electron, resulting in the conversion of a proton into a neutron and the emission of an electron neutrino. Alpha decay emits an alpha particle, composed of two protons and two neutrons, which is essentially a helium-4 nucleus. The key difference lies in the emitted particles: electron capture releases a neutrino, while alpha decay releases a heavy, positively charged helium nucleus.
Effects on Atomic Number and Mass
Electron capture decreases the atomic number by one while leaving the mass number unchanged, as a proton in the nucleus converts into a neutron by capturing an inner orbital electron. Alpha decay reduces both the atomic number by two and the mass number by four, since the nucleus emits an alpha particle consisting of two protons and two neutrons. These nuclear processes significantly alter the element's identity and isotopic composition through distinct changes in proton and neutron counts.
Occurrence and Examples in Nature
Electron capture primarily occurs in proton-rich nuclei, such as potassium-40, where an inner orbital electron is captured by the nucleus, transforming a proton into a neutron and reducing the atomic number by one. Alpha decay is common in heavy elements like uranium-238 and thorium-232, where the nucleus emits an alpha particle (two protons and two neutrons), leading to a decrease in both atomic and mass numbers. While electron capture often stabilizes isotopes with excess protons, alpha decay predominantly affects very heavy, unstable nuclei, contributing to the natural radioactive decay series found in Earth's crust.
Applications and Implications in Science
Electron capture is essential in nuclear medicine for producing specific isotopes used in diagnostic imaging, such as PET scans, due to its ability to transform proton-rich nuclei into neutron-rich ones. Alpha decay plays a critical role in radiometric dating techniques, particularly in dating geological samples and understanding Earth's age by measuring the decay of heavy elements like uranium and thorium. Both processes have profound implications in nuclear physics research, contributing to the study of nuclear stability, elemental transmutation, and fundamental particle interactions.
Summary: Electron Capture vs Alpha Decay
Electron capture involves a proton-rich nucleus capturing an inner orbital electron, converting a proton into a neutron and emitting a neutrino, which decreases the atomic number by one while keeping the mass number unchanged. Alpha decay occurs when an unstable nucleus emits an alpha particle consisting of two protons and two neutrons, reducing the atomic number by two and the mass number by four, often seen in heavy elements like uranium and thorium. Both processes alter the proton-to-neutron ratio to achieve greater nuclear stability but differ fundamentally in emitted particles and their effects on atomic and mass numbers.
Electron Capture Infographic
 libterm.com
libterm.com