Lyophilization, also known as freeze-drying, preserves perishable materials by removing moisture through sublimation, which enhances shelf life and stability without compromising product quality. This process is widely used in pharmaceuticals, food preservation, and biotechnology to maintain the potency and integrity of sensitive substances. Discover how lyophilization can optimize your product storage and delivery by exploring the detailed benefits and applications in the article ahead.
Table of Comparison
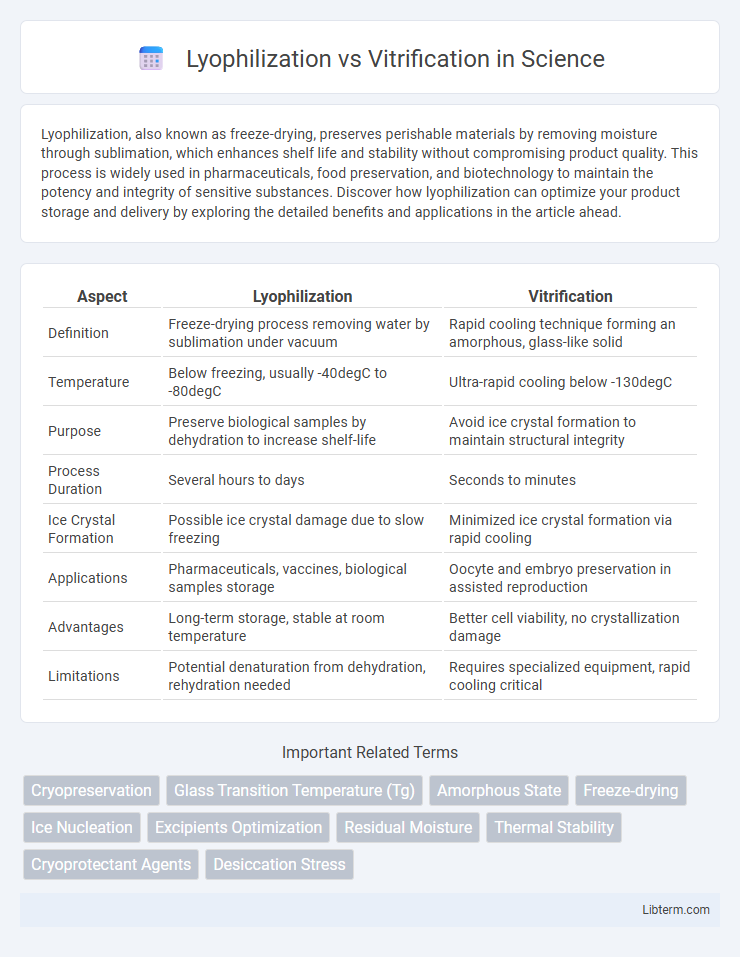
| Aspect | Lyophilization | Vitrification |
|---|---|---|
| Definition | Freeze-drying process removing water by sublimation under vacuum | Rapid cooling technique forming an amorphous, glass-like solid |
| Temperature | Below freezing, usually -40degC to -80degC | Ultra-rapid cooling below -130degC |
| Purpose | Preserve biological samples by dehydration to increase shelf-life | Avoid ice crystal formation to maintain structural integrity |
| Process Duration | Several hours to days | Seconds to minutes |
| Ice Crystal Formation | Possible ice crystal damage due to slow freezing | Minimized ice crystal formation via rapid cooling |
| Applications | Pharmaceuticals, vaccines, biological samples storage | Oocyte and embryo preservation in assisted reproduction |
| Advantages | Long-term storage, stable at room temperature | Better cell viability, no crystallization damage |
| Limitations | Potential denaturation from dehydration, rehydration needed | Requires specialized equipment, rapid cooling critical |
Introduction to Cryopreservation: Lyophilization vs Vitrification
Cryopreservation methods like lyophilization and vitrification are essential for stabilizing biological samples by minimizing ice crystal formation and preserving cellular integrity. Lyophilization involves freeze-drying to remove water via sublimation, making it ideal for long-term storage of pharmaceuticals and bioproducts. Vitrification rapidly cools samples to transform water into an amorphous glass-like state, preventing ice crystallization and ensuring higher viability in reproductive cells and tissues.
Principles of Lyophilization: How It Works
Lyophilization operates by freezing the product and then reducing pressure to allow frozen water to sublimate directly from ice to vapor, preserving the material's structure and bioactivity. This process involves three primary stages: freezing, primary drying (sublimation), and secondary drying (desorption of bound water). By carefully controlling temperature and pressure, lyophilization ensures efficient moisture removal while maintaining product stability, making it ideal for pharmaceuticals, biologics, and food preservation.
Vitrification Explained: A Glass-like State
Vitrification is a cryopreservation technique that transforms biological samples into a glass-like, amorphous solid state without ice crystal formation, preserving cellular structure and function. This process involves ultra-rapid cooling combined with high concentrations of cryoprotectants, preventing crystallization that typically damages cells during freezing. Vitrification is widely used in fertility treatments, organ preservation, and tissue banking due to its superior ability to maintain viability compared to traditional lyophilization or slow freezing methods.
Key Differences Between Lyophilization and Vitrification
Lyophilization involves the sublimation of ice under low temperature and pressure, preserving the structural integrity of biological samples by removing water in solid form, while vitrification rapidly cools samples to transform water into a glass-like amorphous solid without crystallization. Key differences include lyophilization's reliance on phase change from solid to gas and longer processing times versus vitrification's ultra-rapid cooling that prevents ice crystal formation, making it ideal for cryopreservation in reproductive medicine and organ transplantation. Both techniques optimize preservation but differ fundamentally in mechanism, processing speed, and susceptibility to crystallization damage.
Applications in Biomedicine and Pharmaceuticals
Lyophilization preserves biological materials by freeze-drying, widely used for stabilizing vaccines, proteins, and antibiotics to extend shelf life and maintain bioactivity. Vitrification rapidly cools samples into a glass-like state without ice crystal formation, crucial for cryopreserving cells, embryos, and tissues in regenerative medicine and assisted reproductive technologies. Both techniques enhance pharmaceutical formulations by improving stability and efficacy of delicate biomolecules and living cells.
Advantages and Limitations of Lyophilization
Lyophilization offers significant advantages such as enhanced stability, extended shelf life, and preservation of bioactivity for sensitive materials by removing moisture under low temperature and pressure. However, limitations include high operational costs, potential structural damage due to ice crystal formation, and lengthy processing times that may not be suitable for heat-sensitive or complex formulations. Despite these challenges, lyophilization remains a preferred method for pharmaceuticals and biological samples requiring long-term storage and ease of reconstitution.
Benefits and Challenges of Vitrification
Vitrification offers the benefit of preventing ice crystal formation during cryopreservation, thus enhancing cell viability and structural integrity compared to lyophilization. This technique enables rapid cooling rates and avoids the damaging effects of ice, making it ideal for preserving delicate biological samples like embryos and oocytes. Challenges of vitrification include the requirement for high concentrations of cryoprotectants, which can induce cellular toxicity, and the need for precise control of cooling and warming rates to prevent devitrification and sample loss.
Impact on Cellular and Molecular Integrity
Lyophilization preserves cellular and molecular integrity by carefully removing water under low temperature and pressure, minimizing ice crystal formation that can damage cell membranes and proteins. Vitrification prevents ice crystal formation altogether by rapidly cooling cells in cryoprotectant solutions, maintaining the native structure of macromolecules and cellular components. Both techniques aim to enhance cell viability post-preservation, but vitrification typically offers superior protection against molecular denaturation and structural disruption.
Recent Advances in Preservation Technologies
Recent advances in preservation technologies highlight significant improvements in lyophilization and vitrification techniques for biological samples. Enhanced lyophilization methods now incorporate optimized cryoprotectants and controlled shelf-temperature protocols, resulting in higher preservation efficiency and molecular stability. Vitrification innovations emphasize ultra-rapid cooling combined with reduced cryoprotectant toxicity, enabling superior preservation of cellular functions and structural integrity in cryopreserved tissues and embryos.
Choosing the Right Method: Factors to Consider
Choosing between lyophilization and vitrification depends on factors such as the nature of the biological material, desired preservation stability, and end-use application. Lyophilization is preferred for thermostable samples requiring long-term storage with minimal structural changes, while vitrification excels in preserving sensitive cells and tissues by preventing ice crystal formation. Consideration of cost, processing time, and rehydration requirements also plays a critical role in selecting the optimal preservation method.
Lyophilization Infographic
 libterm.com
libterm.com