Hypotonic solutions have a lower concentration of solutes compared to the inside of a cell, causing water to flow into the cell and potentially leading to swelling or bursting. Understanding the effects of hypotonic environments is crucial for medical treatments, hydration therapies, and cell biology studies. Discover how hypotonic solutions impact your body's cells and why this knowledge matters by reading the full article.
Table of Comparison
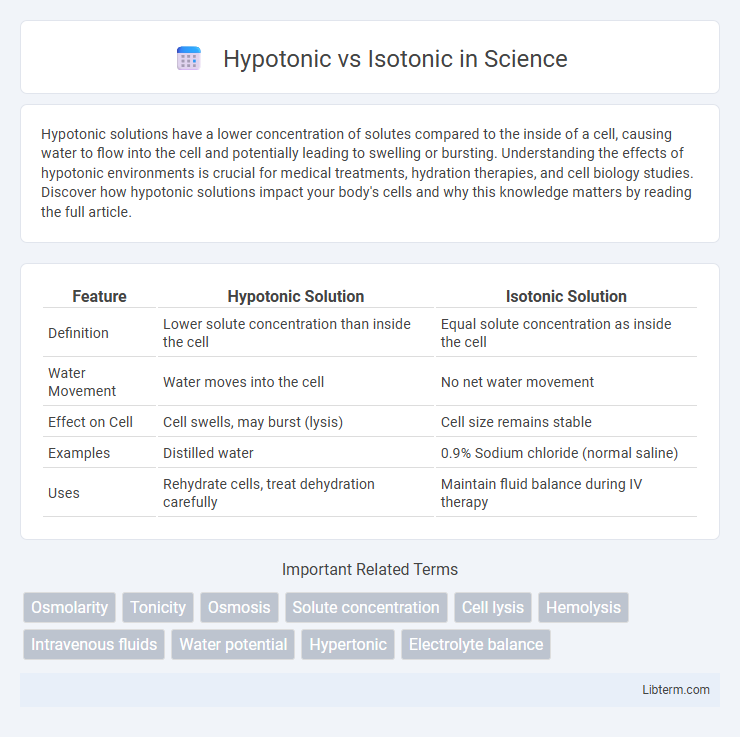
| Feature | Hypotonic Solution | Isotonic Solution |
|---|---|---|
| Definition | Lower solute concentration than inside the cell | Equal solute concentration as inside the cell |
| Water Movement | Water moves into the cell | No net water movement |
| Effect on Cell | Cell swells, may burst (lysis) | Cell size remains stable |
| Examples | Distilled water | 0.9% Sodium chloride (normal saline) |
| Uses | Rehydrate cells, treat dehydration carefully | Maintain fluid balance during IV therapy |
Introduction to Hypotonic and Isotonic Solutions
Hypotonic solutions have a lower concentration of solutes compared to the inside of a cell, causing water to move into the cell and potentially leading to swelling or bursting. Isotonic solutions possess equal solute concentrations inside and outside the cell, promoting balanced water movement and maintaining cell size. These differences are crucial in medical treatments and laboratory settings to manage cell hydration and osmotic pressure effectively.
Definition of Hypotonic Solutions
Hypotonic solutions have a lower concentration of solutes compared to the intracellular fluid, causing water to flow into cells by osmosis. This influx of water can lead to cell swelling or even lysis if the difference is significant. In contrast, isotonic solutions contain equal solute concentrations on both sides of the cell membrane, maintaining cell size and fluid balance.
Definition of Isotonic Solutions
Isotonic solutions have the same solute concentration as the fluid inside cells, allowing for balanced fluid exchange without causing cell shrinkage or swelling. These solutions maintain osmotic equilibrium, making them essential in medical treatments such as intravenous fluid replacement to preserve cellular integrity. Common examples include 0.9% saline and lactated Ringer's solution, which are used to hydrate patients without disrupting electrolyte balance.
Key Differences Between Hypotonic and Isotonic Solutions
Hypotonic solutions have a lower solute concentration compared to the inside of a cell, causing water to flow into the cell and potentially leading to swelling or lysis. Isotonic solutions possess equal solute concentrations inside and outside the cell, resulting in no net water movement and maintaining cell stability. These differences are crucial in medical treatments like intravenous therapy to prevent cellular damage or dehydration.
Cellular Effects of Hypotonic Solutions
Hypotonic solutions cause water to move into cells by osmosis, leading to cell swelling and potential lysis due to lower solute concentration outside the cell compared to the cytoplasm. This osmotic imbalance results in increased intracellular volume and can disrupt cellular function if the swelling is excessive. In contrast, isotonic solutions maintain cell volume and osmotic balance by having equal solute concentration inside and outside the cell, preventing water movement across the membrane.
Cellular Effects of Isotonic Solutions
Isotonic solutions maintain cellular homeostasis by having equal solute concentration inside and outside the cell, preventing net water movement. This equilibrium preserves cell size and shape, ensuring optimal cellular function and integrity. Unlike hypotonic solutions, isotonic solutions avoid cellular swelling or shrinkage, maintaining stable intracellular and extracellular fluid balance.
Examples and Applications in Medicine
Hypotonic solutions, such as 0.45% saline, are used in medicine to treat intracellular dehydration by promoting water influx into cells, beneficial in cases like diabetic ketoacidosis. Isotonic solutions, including 0.9% sodium chloride and lactated Ringer's, maintain fluid balance without causing cell swelling or shrinkage and are commonly applied for volume resuscitation in trauma or surgery. Understanding the distinct osmolarity levels of these fluids guides clinical decisions to optimize patient hydration and electrolyte balance.
Importance in Hydration and Sports Drinks
Hypotonic drinks contain lower concentrations of electrolytes and sugars than body fluids, making them rapidly absorbed for quick rehydration during light exercise or heat exposure. Isotonic drinks match the body's electrolyte and carbohydrate levels, providing balanced hydration and sustained energy release, ideal for moderate to intense sports activities. Understanding the difference ensures athletes optimize fluid replacement, maintain electrolyte balance, and enhance performance and recovery.
Potential Risks and Side Effects
Hypotonic solutions may cause cellular swelling, leading to potential risks such as hyponatremia, cerebral edema, and increased intracranial pressure, especially in patients with brain injuries or electrolyte imbalances. Isotonic solutions generally maintain fluid balance without dramatically altering cell volume but can result in fluid overload, edema, or electrolyte disturbances if administered excessively or in patients with renal or cardiac insufficiency. Careful monitoring of electrolyte levels and fluid status is essential to mitigate adverse effects associated with both hypotonic and isotonic intravenous therapies.
Choosing the Right Solution: Hypotonic vs Isotonic
Choosing the right solution between hypotonic and isotonic fluids depends on the patient's hydration status and cellular needs. Hypotonic solutions, such as 0.45% saline, are ideal for correcting intracellular dehydration by promoting water movement into cells, while isotonic solutions like 0.9% saline maintain extracellular fluid balance without causing fluid shifts across cell membranes. Accurate assessment of electrolyte levels and volume status is crucial to prevent complications such as cellular swelling or fluid overload when administering these intravenous fluids.
Hypotonic Infographic
 libterm.com
libterm.com