Dehydration synthesis is a crucial chemical reaction where molecules combine by removing water, forming bonds essential for building complex compounds like carbohydrates, proteins, and lipids. This process is fundamental in biology for constructing macromolecules from simpler units, aiding in cell structure and function. Explore the rest of this article to understand how dehydration synthesis shapes the molecular architecture of life.
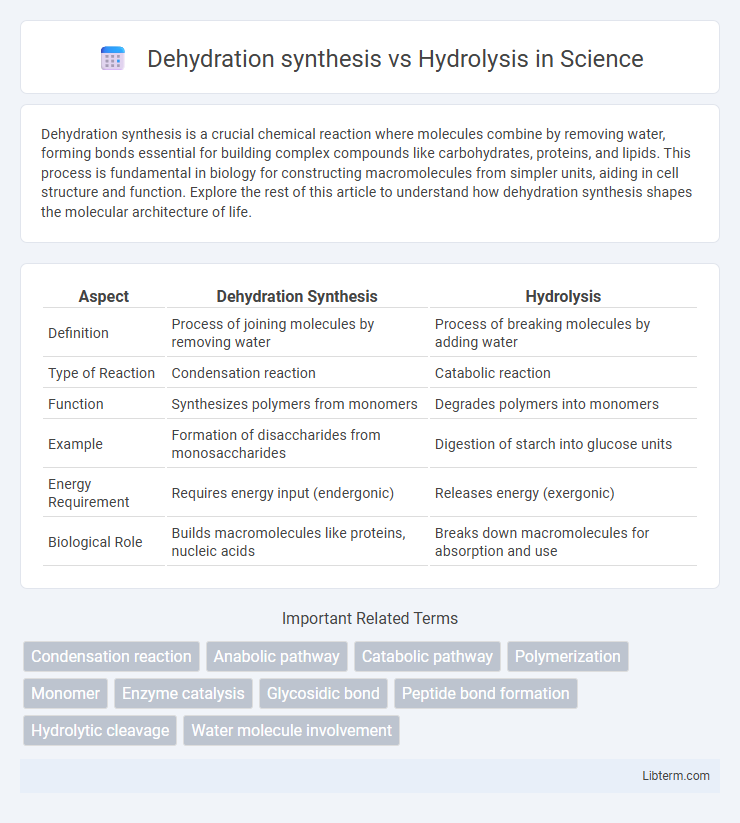
Table of Comparison
| Aspect | Dehydration Synthesis | Hydrolysis |
|---|---|---|
| Definition | Process of joining molecules by removing water | Process of breaking molecules by adding water |
| Type of Reaction | Condensation reaction | Catabolic reaction |
| Function | Synthesizes polymers from monomers | Degrades polymers into monomers |
| Example | Formation of disaccharides from monosaccharides | Digestion of starch into glucose units |
| Energy Requirement | Requires energy input (endergonic) | Releases energy (exergonic) |
| Biological Role | Builds macromolecules like proteins, nucleic acids | Breaks down macromolecules for absorption and use |
Introduction to Dehydration Synthesis and Hydrolysis
Dehydration synthesis is a chemical reaction that forms covalent bonds between monomers by removing a water molecule, playing a critical role in building macromolecules like carbohydrates, proteins, and lipids. Hydrolysis is the reverse process, where water is added to break these covalent bonds and separate polymers into their monomer components. Both reactions are fundamental to cellular metabolism, enabling the dynamic assembly and breakdown of essential biomolecules.
Definition of Dehydration Synthesis
Dehydration synthesis is a chemical reaction that joins two molecules by removing a water molecule, forming a covalent bond between them. This process is essential for building complex macromolecules such as carbohydrates, proteins, and lipids from their monomer units. In contrast, hydrolysis breaks these bonds by adding water, resulting in the decomposition of polymers into monomers.
Definition of Hydrolysis
Hydrolysis is a chemical process that breaks down complex molecules into simpler units by adding water, effectively cleaving chemical bonds. This reaction is essential in biological systems for digestion and energy release, contrasting with dehydration synthesis, which forms bonds by removing water. Hydrolysis facilitates the breakdown of polymers like proteins, carbohydrates, and lipids into monomers for cellular use.
Key Differences Between Dehydration Synthesis and Hydrolysis
Dehydration synthesis involves the removal of a water molecule to bond two monomers into a polymer, whereas hydrolysis uses water to break those bonds, splitting polymers back into monomers. Enzymes like synthases catalyze dehydration synthesis, while hydrolases facilitate hydrolysis reactions. The processes are essentially opposite, with dehydration synthesis building complex molecules and hydrolysis breaking them down for digestion and cellular processes.
Chemical Reactions: Mechanisms and Processes
Dehydration synthesis involves the chemical reaction where two molecules covalently bond through the removal of a water molecule, often facilitated by enzymes like synthases. Hydrolysis, conversely, is the cleavage of chemical bonds in molecules by the insertion of a water molecule, typically catalyzed by hydrolase enzymes. These processes are fundamental in biological systems for building complex molecules such as polysaccharides, lipids, and proteins, as well as breaking them down for energy and recycling.
Biological Significance in Cells
Dehydration synthesis and hydrolysis are crucial biochemical reactions that regulate macromolecule assembly and breakdown in cells. Dehydration synthesis forms polymers like proteins and nucleic acids by removing water molecules, enabling the storage of energy and structural integrity essential for cellular functions. Hydrolysis reverses this process, breaking down complex molecules into monomers to release energy and provide building blocks for cellular metabolism and repair.
Examples in Carbohydrate Metabolism
Dehydration synthesis in carbohydrate metabolism involves the formation of disaccharides like sucrose and polysaccharides such as starch and glycogen by linking monosaccharides with glycosidic bonds while releasing water molecules. Hydrolysis breaks down these complex carbohydrates into simple sugars during digestion or cellular respiration, with enzymes like amylase converting starch into maltose and then glucose. These contrasting processes are critical for energy storage and release, enabling efficient metabolic regulation in organisms.
Role in Protein and Lipid Formation
Dehydration synthesis plays a crucial role in protein formation by linking amino acids through peptide bonds, while in lipids, it forms ester bonds between glycerol and fatty acids. Hydrolysis, in contrast, breaks these bonds by adding water, enabling the breakdown of proteins into amino acids and lipids into glycerol and fatty acids. This dynamic balance between dehydration synthesis and hydrolysis is essential for maintaining cellular functions and metabolic processes.
Importance in Digestion and Energy Release
Dehydration synthesis is essential in digestion for building complex molecules like carbohydrates, proteins, and lipids by removing water to form bonds, facilitating nutrient storage and structural functions. Hydrolysis is crucial for breaking down these macromolecules into their monomers during digestion, releasing energy that cells use for metabolic processes. The dynamic balance between dehydration synthesis and hydrolysis maintains metabolic homeostasis and supports efficient energy release for cellular activities.
Summary: Comparing Dehydration Synthesis and Hydrolysis
Dehydration synthesis and hydrolysis are fundamental biochemical processes with opposite mechanisms: dehydration synthesis forms covalent bonds by removing a water molecule, while hydrolysis breaks these bonds by adding water. Dehydration synthesis is essential in building complex macromolecules like proteins, lipids, and polysaccharides, whereas hydrolysis is crucial for breaking down these macromolecules into monomers for cellular use. Both processes regulate metabolic pathways and maintain cellular homeostasis by controlling the assembly and disassembly of biomolecules.
Dehydration synthesis Infographic
 libterm.com
libterm.com