Neutral tones create a calm and balanced atmosphere in any space, offering versatility that complements a wide range of styles and colors. These shades work well to highlight your furnishings and artwork without overpowering the room's overall design. Explore how incorporating neutral colors can transform your space by reading the full article.
Table of Comparison
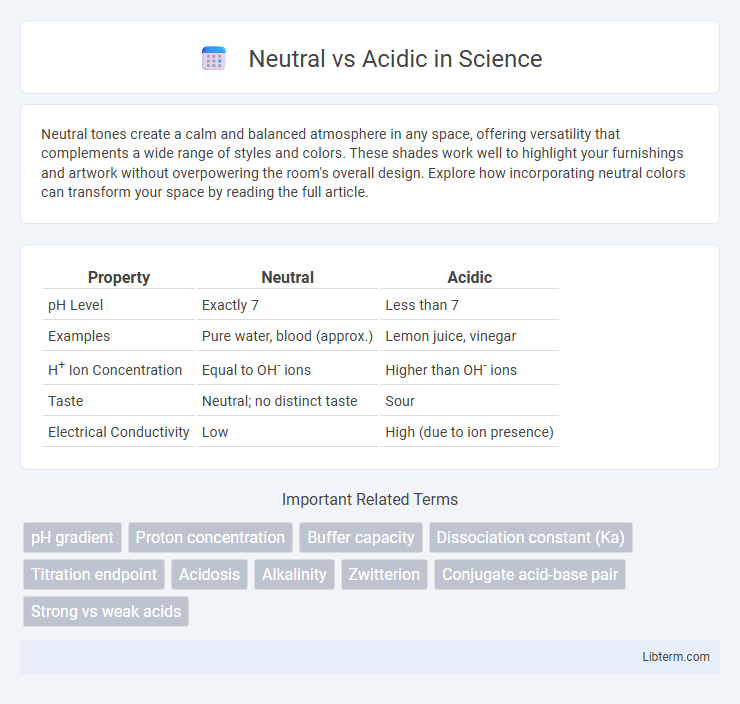
| Property | Neutral | Acidic |
|---|---|---|
| pH Level | Exactly 7 | Less than 7 |
| Examples | Pure water, blood (approx.) | Lemon juice, vinegar |
| H+ Ion Concentration | Equal to OH- ions | Higher than OH- ions |
| Taste | Neutral; no distinct taste | Sour |
| Electrical Conductivity | Low | High (due to ion presence) |
Understanding pH: The Basics of Neutral and Acidic
pH measures the concentration of hydrogen ions in a solution, ranging from 0 to 14, with 7 considered neutral. Solutions with a pH less than 7 are acidic, indicating higher hydrogen ion concentration, while those above 7 are basic or alkaline, reflecting lower hydrogen ion levels. Understanding pH is crucial for applications in chemistry, biology, and environmental science, as it affects chemical reactions, biological processes, and ecosystem health.
Key Differences Between Neutral and Acidic Substances
Neutral substances have a pH of exactly 7, indicating a balance between hydrogen ions (H+) and hydroxide ions (OH-), resulting in neither acidic nor basic properties. Acidic substances possess a pH less than 7, characterized by a higher concentration of hydrogen ions, which contributes to their sour taste and corrosive nature. Key differences include their reaction with indicators--neutral substances do not change the color of litmus paper, whereas acids turn blue litmus paper red--and their ability to neutralize bases in chemical reactions.
The Science Behind Acidity and Neutrality
Acidity and neutrality are determined by the concentration of hydrogen ions (H+) in a solution, measured on the pH scale where values below 7 indicate acidity and exactly 7 signifies neutrality. Acids release hydrogen ions, increasing the concentration of H+, while neutral substances maintain a balanced concentration of hydrogen and hydroxide ions (OH-). The molecular structure and ionization mechanisms of substances define their acidic or neutral properties, influencing chemical reactions and biological processes.
Common Examples of Neutral and Acidic Solutions
Neutral solutions commonly include pure water and saline solutions, both maintaining a pH of around 7, indicating neither acidic nor basic properties. Acidic solutions are frequently represented by substances such as vinegar, with acetic acid, and lemon juice, containing citric acid, typically showcasing a pH below 7. These examples illustrate the fundamental pH difference, where neutral solutions offer a balance of hydrogen ions and hydroxide ions, while acidic solutions exhibit higher concentrations of hydrogen ions.
Effects of Acidity and Neutrality in Everyday Life
Acidity influences corrosion rates in metals, with acidic environments accelerating rust formation on iron and steel objects, while neutral pH levels tend to slow this process. Skin health is affected as well, with slightly acidic skin pH (around 5.5) maintaining the natural barrier against bacteria, whereas neutral or alkaline conditions can lead to dryness and increased susceptibility to infections. In agriculture, soil pH significantly impacts nutrient availability, with acidic soils limiting essential minerals like phosphorus, and neutral soils promoting optimal plant growth and microbial activity.
Measuring pH: Tools and Techniques
Measuring pH accurately involves using tools such as pH meters, litmus paper, and pH indicator strips, each designed to detect hydrogen ion concentration in solutions. A pH meter provides precise digital readings suitable for laboratory and industrial applications, while litmus paper and indicator strips offer quick, qualitative assessments of whether a solution is neutral (pH 7), acidic (pH below 7), or basic (pH above 7). Calibration of pH meters with standard buffer solutions ensures accurate measurement, critical for processes in chemistry, agriculture, and environmental monitoring.
Health Impacts: Acidic vs Neutral Environments
Acidic environments in the body can lead to increased inflammation, contributing to chronic diseases such as arthritis and cardiovascular issues. Maintaining a neutral pH balance supports optimal enzymatic function and enhances nutrient absorption, promoting overall health. Excessive acidity may also disrupt bone density by leaching calcium, increasing the risk of osteoporosis.
Role of Neutral and Acidic Solutions in Industry
Neutral solutions serve as essential solvents and cleaning agents in industries like electronics and pharmaceuticals due to their non-reactive nature, ensuring material stability and product purity. Acidic solutions are widely used in metal processing, chemical manufacturing, and pH regulation, facilitating processes such as etching, catalysis, and neutralization of alkaline waste. Industrial applications prioritize precise pH control to optimize reaction rates, product quality, and environmental safety.
Strategies to Balance Acidity and Neutrality
Balancing acidity and neutrality in soil requires precise pH management through amendments like lime to raise pH or sulfur to lower it, optimizing nutrient availability for plant growth. Regular soil testing informs the appropriate quantity and timing of these amendments, promoting a stable environment that supports microbial activity and nutrient absorption. Employing organic matter such as compost enhances buffering capacity, further stabilizing pH fluctuations and maintaining an ideal balance for diverse crops.
Frequently Asked Questions on Neutral vs Acidic
Neutral substances have a pH of exactly 7, indicating a balanced concentration of hydrogen ions (H+) and hydroxide ions (OH-), while acidic substances have a pH less than 7, characterized by a higher concentration of H+ ions. Common questions involve the impact of neutral versus acidic environments on chemical reactions, biological systems, and soil quality, where acidic soils can affect nutrient availability and plant growth differently than neutral soils. Understanding the pH scale, methods for measuring pH, and the effects of neutral and acidic conditions is essential for applications in chemistry, agriculture, and health sciences.
Neutral Infographic
 libterm.com
libterm.com