Competitive markets drive innovation and efficiency, pushing businesses to constantly improve their products and services to meet consumer demands. Understanding the dynamics of competition can help you identify opportunities for growth and stay ahead in your industry. Explore the full article to uncover strategies that enhance your competitive edge.
Table of Comparison
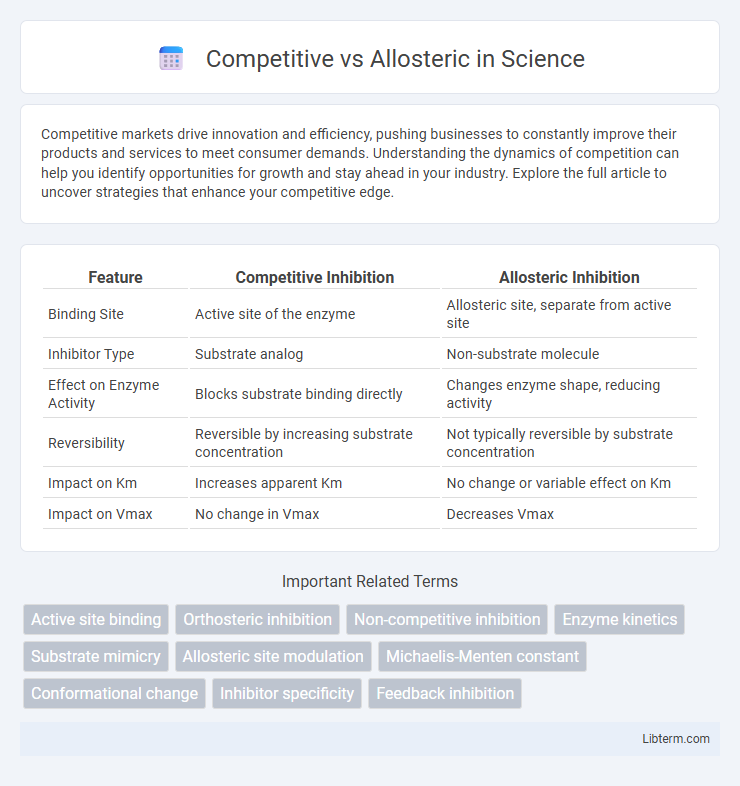
| Feature | Competitive Inhibition | Allosteric Inhibition |
|---|---|---|
| Binding Site | Active site of the enzyme | Allosteric site, separate from active site |
| Inhibitor Type | Substrate analog | Non-substrate molecule |
| Effect on Enzyme Activity | Blocks substrate binding directly | Changes enzyme shape, reducing activity |
| Reversibility | Reversible by increasing substrate concentration | Not typically reversible by substrate concentration |
| Impact on Km | Increases apparent Km | No change or variable effect on Km |
| Impact on Vmax | No change in Vmax | Decreases Vmax |
Understanding Enzyme Inhibition
Competitive inhibition occurs when an inhibitor competes directly with the substrate for binding to the enzyme's active site, which increases the apparent Km without affecting Vmax. Allosteric inhibition involves the binding of an inhibitor to a site other than the active site, causing conformational changes that reduce enzyme activity and decrease Vmax without changing Km. Understanding these distinct mechanisms is crucial for designing drugs that precisely modulate enzyme function in biochemical pathways.
Definition of Competitive Inhibition
Competitive inhibition occurs when a molecule structurally similar to the substrate binds to the enzyme's active site, preventing the actual substrate from attaching. This reversible binding blocks the catalytic activity by directly competing for the same binding location on the enzyme. The key feature of competitive inhibition is that increasing substrate concentration can overcome the inhibition, restoring enzyme activity.
Mechanism of Competitive Inhibition
Competitive inhibition involves an inhibitor molecule binding directly to the active site of an enzyme, blocking substrate attachment and preventing catalytic activity. This reversible binding depends on the concentration of both substrate and inhibitor, allowing substrate molecules to outcompete the inhibitor at high substrate levels. The mechanism alters enzyme kinetics by increasing the apparent Km without affecting the maximum velocity (Vmax), distinguishing it from allosteric inhibition, where effectors bind to alternate sites to induce conformational changes.
Definition of Allosteric Inhibition
Allosteric inhibition occurs when an inhibitor binds to a site other than the enzyme's active site, causing a conformational change that reduces enzyme activity. This differs from competitive inhibition, where the inhibitor directly competes with the substrate for active site binding. Allosteric inhibitors enable regulation of enzyme function through reversible structural modifications without blocking substrate access.
Mechanism of Allosteric Inhibition
Allosteric inhibition occurs when an inhibitor binds to a site distinct from the enzyme's active site, inducing a conformational change that reduces enzymatic activity. This mechanism alters the enzyme's shape, decreasing substrate affinity without directly competing with the substrate. Unlike competitive inhibition, allosteric inhibitors modulate enzyme function by stabilizing inactive forms or disrupting essential conformational dynamics critical for catalysis.
Structural Differences: Competitive vs Allosteric
Competitive inhibitors bind directly to the active site of an enzyme, structurally resembling the substrate to block substrate access, whereas allosteric inhibitors attach to a distinct regulatory site separate from the active site. This binding induces conformational changes in the enzyme's structure, altering its activity without directly competing with the substrate. Structural differences in binding sites and the resultant enzyme conformations define the specificity and mechanism of inhibition between competitive and allosteric modulators.
Impact on Enzyme Kinetics
Competitive inhibitors increase the apparent Km of an enzyme without affecting the Vmax, as they compete with the substrate for the active site, reducing substrate binding affinity. Allosteric inhibitors decrease the Vmax by binding to sites other than the active site, inducing conformational changes that reduce enzyme activity without altering substrate affinity. The distinct impacts on enzyme kinetics enable differentiation between competitive and allosteric inhibition through Michaelis-Menten and Lineweaver-Burk plots.
Physiological and Pharmaceutical Relevance
Competitive inhibitors bind reversibly to the active site of enzymes, directly competing with substrates and often modulating metabolic pathways important in drug metabolism and neurotransmission. Allosteric inhibitors bind to distinct regulatory sites, inducing conformational changes that affect enzyme activity, offering higher selectivity and reduced side effects in pharmaceutical drug design. Physiologically, allosteric regulation allows fine-tuning of enzyme function in response to cellular signals, while competitive inhibition provides rapid, reversible control of enzymatic reactions critical for maintaining metabolic balance.
Advantages and Disadvantages of Each Inhibition Type
Competitive inhibition offers the advantage of reversibility and specificity, as inhibitors bind directly to the active site, allowing substrate concentration to overcome inhibition; however, its effectiveness diminishes at high substrate levels. Allosteric inhibition can regulate enzyme activity more effectively by binding to sites distinct from the active site, enabling conformational changes that modulate function and often resulting in greater control over metabolic pathways. A disadvantage of allosteric inhibitors is potential complexity and variable effects depending on enzyme conformation, which can complicate drug design and predictability of inhibition.
Key Takeaways: Competitive vs Allosteric Inhibition
Competitive inhibition involves molecules binding to the active site of an enzyme, directly competing with the substrate and increasing Km without affecting Vmax. Allosteric inhibition occurs when inhibitors bind to a site other than the active site, causing conformational changes that decrease enzyme activity and reduce Vmax without changing Km. Understanding these mechanisms is crucial for drug design, as competitive inhibitors often require higher substrate concentrations to be effective, whereas allosteric inhibitors modulate enzyme function more subtly.
Competitive Infographic
 libterm.com
libterm.com