Adsorbing is the process by which molecules, atoms, or ions adhere to the surface of a material, creating a thin film or layer. This phenomenon plays a critical role in various applications including water purification, air filtration, and chemical sensors, enhancing efficiency and selectivity. Discover how adsorbing can impact your projects by exploring the detailed mechanisms and practical uses in the rest of this article.
Table of Comparison
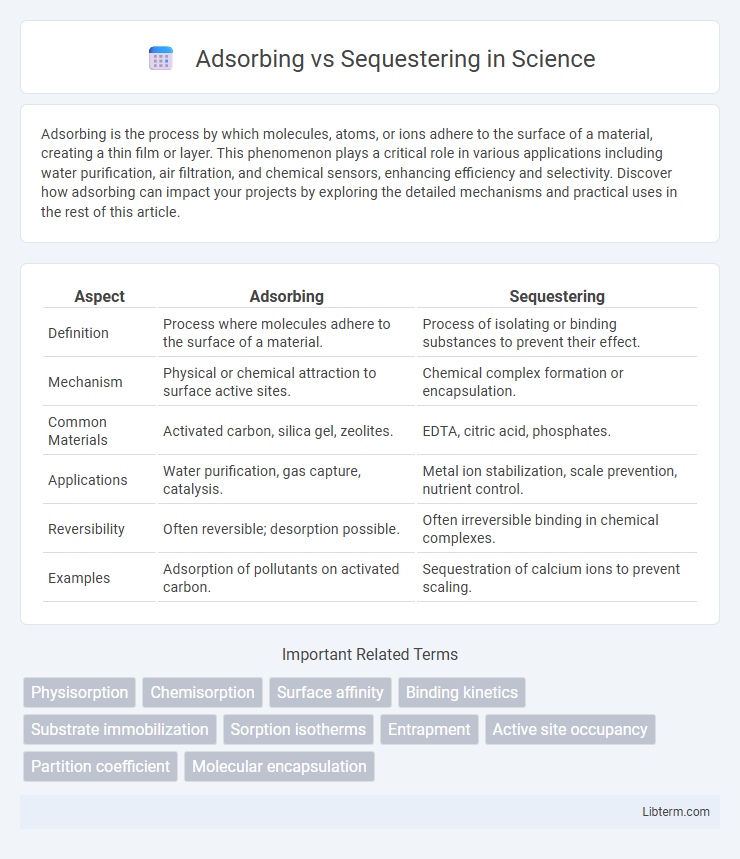
| Aspect | Adsorbing | Sequestering |
|---|---|---|
| Definition | Process where molecules adhere to the surface of a material. | Process of isolating or binding substances to prevent their effect. |
| Mechanism | Physical or chemical attraction to surface active sites. | Chemical complex formation or encapsulation. |
| Common Materials | Activated carbon, silica gel, zeolites. | EDTA, citric acid, phosphates. |
| Applications | Water purification, gas capture, catalysis. | Metal ion stabilization, scale prevention, nutrient control. |
| Reversibility | Often reversible; desorption possible. | Often irreversible binding in chemical complexes. |
| Examples | Adsorption of pollutants on activated carbon. | Sequestration of calcium ions to prevent scaling. |
Introduction to Adsorbing and Sequestering
Adsorbing refers to the process where molecules adhere only to the surface of a material, creating a film or layer without penetrating the substance. Sequestering involves the formation of stable complexes with specific ions or molecules, effectively isolating them to prevent unwanted reactions or interactions. Both processes are critical in environmental science and industrial applications for contaminant removal and chemical stabilization.
Defining Adsorption: Mechanisms and Materials
Adsorption involves the accumulation of molecules from a gas or liquid onto the surface of a solid or liquid, driven primarily by physical or chemical interactions such as van der Waals forces, hydrogen bonding, or covalent bonding. Common adsorbent materials include activated carbon, zeolites, silica gel, and metal-organic frameworks (MOFs), each offering high surface area and specific functional groups to enhance adsorption capacity and selectivity. This surface-based retention differentiates adsorption from sequestration, which typically refers to stable, long-term entrapment within a matrix or phase, rather than surface adherence.
Understanding Sequestration: Processes and Applications
Sequestration involves capturing and storing substances, such as carbon dioxide or pollutants, to prevent their release into the environment, typically through chemical or biological processes. This method is essential in climate change mitigation, where carbon sequestration traps CO2 in forests, soil, or geological formations to reduce atmospheric levels. Applications extend to wastewater treatment and soil remediation, where sequestration immobilizes contaminants, enhancing environmental safety and sustainability.
Key Differences Between Adsorbing and Sequestering
Adsorbing involves the adhesion of molecules or particles onto a surface, creating a concentrated layer without chemical alteration, while sequestering refers to the chemical binding or complexation of substances to immobilize or isolate them in solution. Adsorption is a physical process often used in filtration and purification systems, whereas sequestering is commonly applied in water treatment and chemical stabilization to prevent unwanted reactions. The key difference lies in adsorption's surface-based mechanism versus sequestering's chemical interaction and complex formation.
Adsorption in Environmental Remediation
Adsorption plays a crucial role in environmental remediation by capturing contaminants on the surface of solid adsorbents like activated carbon, zeolites, and biochar. This process effectively removes pollutants such as heavy metals, organic compounds, and dyes from water and air, enabling cleaner ecosystems. Adsorption's efficiency depends on the adsorbent's surface area, pore size, and chemical properties, making tailored materials essential for targeted contaminant removal.
Sequestration in Carbon Capture and Storage
Sequestration in Carbon Capture and Storage (CCS) involves the long-term isolation of CO2 from the atmosphere by storing it underground in geological formations such as depleted oil and gas fields or deep saline aquifers. Unlike adsorption, which captures carbon temporarily on surfaces or materials, sequestration permanently contains carbon, preventing its release back into the environment. Effective sequestration is critical for mitigating climate change by reducing atmospheric carbon dioxide concentrations over decades to millennia.
Efficiency Comparison: Adsorbing vs Sequestering
Adsorbing processes typically exhibit higher efficiency in capturing contaminants due to direct surface interaction and greater adsorption capacity of materials like activated carbon. Sequestering relies on chemical stabilization or encapsulation which may reduce bioavailability but often results in slower kinetics and lower removal rates. Overall, adsorbing demonstrates superior immediate contaminant reduction, while sequestering offers long-term containment with varied efficiency based on matrix and contaminant type.
Material Science Innovations: Adsorbents and Sequestrants
In material science innovations, adsorbents excel by capturing molecules on their surface through physical or chemical interactions, enhancing applications in gas purification and water treatment. Sequestrants operate by chemically binding contaminants within a matrix, effectively isolating harmful substances to prevent environmental contamination. Advances in porous materials and functionalized polymers significantly improve the efficiency and selectivity of both adsorbing and sequestering agents.
Industrial Applications of Adsorption and Sequestration
Adsorption in industrial applications involves the adherence of molecules or particles onto solid surfaces, widely used in gas purification, wastewater treatment, and catalyst recovery, enhancing process efficiency and product quality. Sequestration, mainly applied in environmental management, focuses on capturing and isolating contaminants or greenhouse gases like CO2 to reduce environmental impact and carbon footprints. Both adsorption and sequestration technologies are critical in industries aiming for sustainable operations, with adsorption favoring reversible surface interactions and sequestration emphasizing long-term containment.
Future Trends in Adsorbing and Sequestering Technologies
Future trends in adsorbing technologies emphasize the development of advanced nanomaterials and bio-inspired adsorbents that offer higher surface area and selectivity for targeted pollutant removal. Sequestering technologies are increasingly integrating smart encapsulation methods and responsive materials capable of controlled release and enhanced stability under varying environmental conditions. Innovations in both fields focus on sustainability, scalability, and real-time monitoring to improve efficiency in water treatment, carbon capture, and waste management applications.
Adsorbing Infographic
 libterm.com
libterm.com