Element plays a fundamental role in chemistry, representing a pure substance consisting of only one type of atom. Understanding the properties and behavior of different elements is crucial for grasping how materials interact and combine in various chemical reactions. Explore the rest of the article to discover how elements impact everyday life and scientific advancements.
Table of Comparison
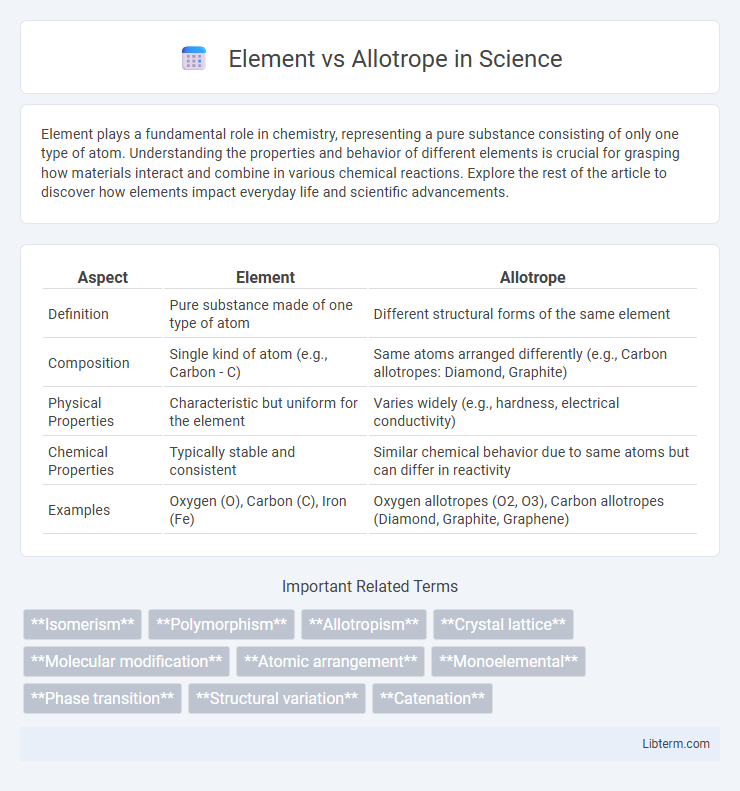
| Aspect | Element | Allotrope |
|---|---|---|
| Definition | Pure substance made of one type of atom | Different structural forms of the same element |
| Composition | Single kind of atom (e.g., Carbon - C) | Same atoms arranged differently (e.g., Carbon allotropes: Diamond, Graphite) |
| Physical Properties | Characteristic but uniform for the element | Varies widely (e.g., hardness, electrical conductivity) |
| Chemical Properties | Typically stable and consistent | Similar chemical behavior due to same atoms but can differ in reactivity |
| Examples | Oxygen (O), Carbon (C), Iron (Fe) | Oxygen allotropes (O2, O3), Carbon allotropes (Diamond, Graphite, Graphene) |
Understanding the Basics: Element vs Allotrope
An element is a pure chemical substance consisting of atoms with the same number of protons, defining its unique atomic structure, while allotropes are different structural forms of the same element, exhibiting distinct physical and chemical properties. Carbon, for example, exists as allotropes such as diamond, graphite, and graphene, each with unique bonding arrangements and characteristics despite having the same elemental composition. Understanding the differentiation between elements and their allotropes is essential for exploring material properties and chemical behavior in various scientific fields.
Defining Elements in Chemistry
Elements in chemistry are defined as pure substances consisting of only one type of atom, characterized by a specific number of protons in their atomic nuclei, known as the atomic number. Each element exhibits unique chemical and physical properties due to its distinct atomic structure. Unlike allotropes, which are different structural forms of the same element, an element represents the fundamental building block of matter in the periodic table.
What Are Allotropes?
Allotropes are different structural forms of the same element, where atoms are bonded together in distinct ways, resulting in varied physical and chemical properties. Carbon, for example, exists as allotropes such as diamond, graphite, and graphene, each exhibiting unique hardness, conductivity, and appearance. These variations arise because the atomic arrangement and bonding affect the element's overall characteristics.
Key Differences Between Elements and Allotropes
Elements consist of atoms with the same number of protons, representing a pure substance defined by its atomic number, while allotropes are different structural forms of the same element exhibiting distinct physical and chemical properties. Elements cannot be broken down into simpler substances by chemical means, whereas allotropes arise from variations in atomic arrangement or bonding within an element, such as carbon existing as diamond, graphite, and graphene. These key differences impact their reactivity, hardness, electrical conductivity, and other material characteristics crucial in scientific and industrial applications.
Examples of Elements and Their Allotropes
Carbon is a prime example of an element with distinct allotropes, including graphite, diamond, and graphene, each exhibiting unique physical properties due to different atomic arrangements. Oxygen exists as two allotropes: O2 (dioxygen), essential for respiration, and O3 (ozone), which protects against ultraviolet radiation in the stratosphere. Sulfur's common allotropes include rhombic sulfur (S8) and monoclinic sulfur, differentiating in crystal structure and stability at varying temperatures.
How Allotropes Form from Elements
Allotropes form when atoms of a single element bond together in different structural arrangements, resulting in distinct physical and chemical properties. Variations in atomic connectivity and molecular geometry, such as carbon atoms forming diamond's tetrahedral lattice or graphite's layered sheets, illustrate how allotropes emerge from the same element. The energy conditions and bonding types influence the stability and transformation between different allotropes.
Physical and Chemical Properties: Element vs Allotrope
An element is a pure substance consisting of only one type of atom, defined by its atomic number, whereas allotropes are different structural forms of the same element with distinct physical and chemical properties. Physical differences between allotropes include variations in density, color, hardness, and melting point, while chemical properties can differ due to differences in atomic bonding and molecular arrangements. For example, carbon allotropes such as graphite and diamond have identical elemental composition but exhibit vastly different hardness and electrical conductivity due to their unique atomic structures.
Importance of Allotropes in Science and Industry
Allotropes are different structural forms of the same element, exhibiting unique physical and chemical properties that make them crucial in various scientific and industrial applications. For example, carbon allotropes like diamond and graphite serve distinct roles--diamonds in cutting tools and graphite in lubricants and batteries--highlighting the importance of allotropes in material science and technology development. Understanding and manipulating allotropes enable advancements in nanotechnology, electronics, and pharmaceuticals, driving innovation across multiple industries.
Real-World Applications of Allotropes
Allotropes exhibit unique physical and chemical properties that make them invaluable in real-world applications across various industries. For example, diamond, an allotrope of carbon, is widely used in cutting, grinding, and drilling due to its exceptional hardness, while graphite, another carbon allotrope, serves as a crucial lubricant and electrode material in batteries. These distinct allotropes allow for tailored solutions in manufacturing, electronics, and materials science, demonstrating their practical importance beyond the elemental form.
Summary: Element vs Allotrope Comparison
An element is a pure substance consisting of one type of atom defined by its atomic number, while allotropes are different structural forms of the same element with distinct physical and chemical properties. Carbon, for example, exists as allotropes such as diamond, graphite, and graphene, each exhibiting unique characteristics despite being composed solely of carbon atoms. Understanding the differences between elements and their allotropes is crucial for applications in materials science, chemistry, and nanotechnology.
Element Infographic
 libterm.com
libterm.com