Photosynthesis is the process by which green plants, algae, and some bacteria convert sunlight, carbon dioxide, and water into glucose and oxygen, providing essential energy for life on Earth. This natural mechanism not only sustains plant growth but also maintains atmospheric oxygen levels vital for all aerobic organisms. Explore the rest of this article to understand how photosynthesis influences ecosystems and your daily life.
Table of Comparison
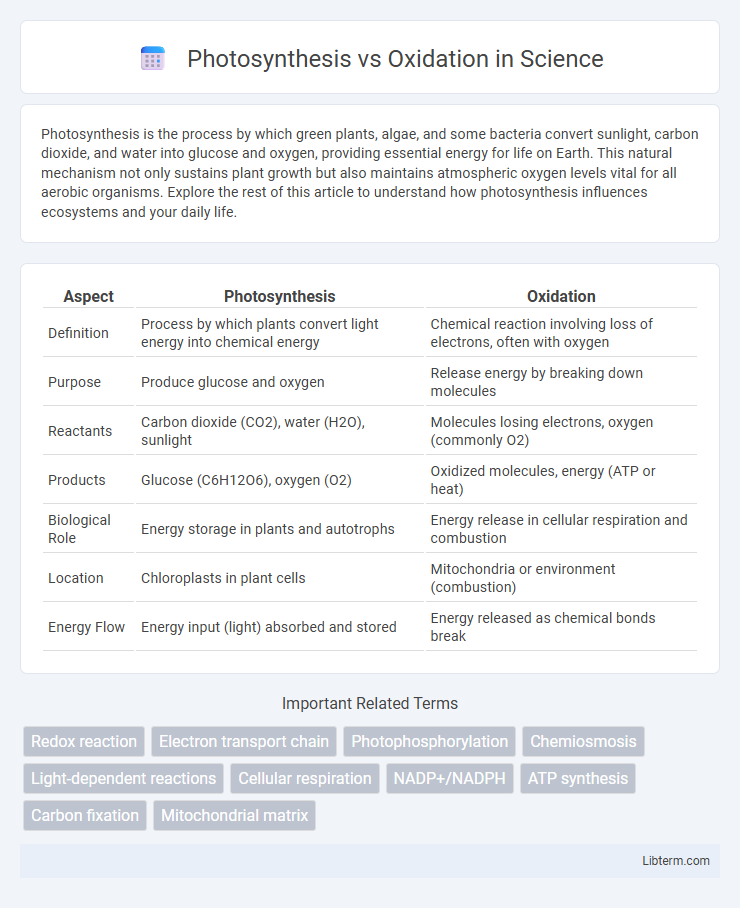
| Aspect | Photosynthesis | Oxidation |
|---|---|---|
| Definition | Process by which plants convert light energy into chemical energy | Chemical reaction involving loss of electrons, often with oxygen |
| Purpose | Produce glucose and oxygen | Release energy by breaking down molecules |
| Reactants | Carbon dioxide (CO2), water (H2O), sunlight | Molecules losing electrons, oxygen (commonly O2) |
| Products | Glucose (C6H12O6), oxygen (O2) | Oxidized molecules, energy (ATP or heat) |
| Biological Role | Energy storage in plants and autotrophs | Energy release in cellular respiration and combustion |
| Location | Chloroplasts in plant cells | Mitochondria or environment (combustion) |
| Energy Flow | Energy input (light) absorbed and stored | Energy released as chemical bonds break |
Introduction to Photosynthesis and Oxidation
Photosynthesis is the biological process in which green plants, algae, and certain bacteria convert light energy into chemical energy stored in glucose while releasing oxygen as a byproduct. Oxidation involves the loss of electrons from a molecule, atom, or ion, often associated with the release of energy through the breakdown of organic compounds. Both processes are fundamental to the energy cycle in living organisms, with photosynthesis capturing energy and oxidation facilitating energy release.
Defining Photosynthesis: Process and Purpose
Photosynthesis is a biochemical process in plants, algae, and certain bacteria that converts light energy into chemical energy stored as glucose, using carbon dioxide and water. This process primarily occurs in chloroplasts, where chlorophyll captures sunlight to drive a series of reactions that produce oxygen as a byproduct. The purpose of photosynthesis is to create organic compounds that serve as energy sources for autotrophic organisms and form the base of most food chains.
Understanding Oxidation: Mechanisms and Importance
Oxidation is a chemical process where molecules lose electrons, often involving oxygen, playing a crucial role in cellular respiration by converting nutrients into energy. This mechanism drives the metabolism in living organisms, facilitating the breakdown of glucose to produce ATP, the primary energy currency. Understanding oxidation helps reveal how energy transfer sustains life functions and contrasts with photosynthesis, which stores energy by capturing light through electron gain.
Key Chemical Reactions in Photosynthesis
Photosynthesis primarily involves the light-dependent reactions where chlorophyll absorbs sunlight to convert water (H2O) into oxygen (O2), protons, and electrons through photolysis, generating ATP and NADPH. The Calvin cycle then uses ATP and NADPH to fix carbon dioxide (CO2) into glucose (C6H12O6) via a series of enzyme-catalyzed reactions involving ribulose bisphosphate carboxylase/oxygenase (RuBisCO). In contrast, oxidation involves the breakdown of glucose during cellular respiration, where glucose is oxidized to carbon dioxide and water, releasing energy as ATP through electron transport chains and oxidative phosphorylation.
Core Steps of Oxidation Reactions
Oxidation reactions involve the loss of electrons from molecules, a process central to cellular respiration and energy production. Core steps include the removal of hydrogen atoms, the transfer of electrons to electron carriers like NAD+ or FAD, and the eventual passage of electrons through the electron transport chain to generate ATP. Unlike photosynthesis, which captures light energy to synthesize glucose, oxidation reactions break down glucose to release stored energy.
Energy Conversion: From Sunlight to Chemical and Chemical to Heat
Photosynthesis converts sunlight into chemical energy by synthesizing glucose through chlorophyll-containing cells in plants, capturing solar energy to fuel biological processes. Oxidation involves breaking down this glucose in cellular respiration, releasing chemical energy stored in bonds and converting it into usable ATP while dissipating excess energy as heat. These energy conversions illustrate the transformation from solar to chemical energy and from chemical energy to thermal energy essential for living organisms.
Major Biological Roles: Building vs Breaking Molecules
Photosynthesis primarily serves a building function by converting carbon dioxide and water into glucose and oxygen, essential for energy storage and plant growth. Oxidation focuses on breaking down molecules, such as glucose, to release energy necessary for cellular activities in both plants and animals. These processes are complementary, with photosynthesis synthesizing organic molecules and oxidation degrading them to sustain metabolism.
Photosynthesis vs Oxidation: Similarities and Differences
Photosynthesis and oxidation both involve electron transfer processes essential for energy conversion in living organisms. Photosynthesis captures solar energy to convert carbon dioxide and water into glucose and oxygen, while oxidation breaks down glucose to release energy in the form of ATP by combining it with oxygen. Despite their contrasting roles--photosynthesis as an energy-storing process and oxidation as an energy-releasing one--both are interconnected in the global carbon and energy cycles.
Environmental Impact of Each Process
Photosynthesis significantly reduces atmospheric carbon dioxide by converting it into oxygen and organic compounds, playing a crucial role in mitigating climate change and supporting ecosystems. Oxidation processes, especially those involving fossil fuel combustion, contribute to increased greenhouse gas emissions and air pollution, exacerbating global warming and harming biodiversity. The balance between photosynthesis and oxidation directly influences the Earth's carbon cycle and overall environmental health.
Conclusion: Balancing Photosynthesis and Oxidation in Nature
Photosynthesis and oxidation are complementary biochemical processes essential for energy flow and carbon cycling in ecosystems. Photosynthesis captures solar energy to convert carbon dioxide and water into glucose and oxygen, while oxidation breaks down glucose to release energy and produce carbon dioxide. Balancing these processes maintains atmospheric oxygen levels, supports plant growth, and sustains life by regulating the energy and carbon exchange in natural systems.
Photosynthesis Infographic
 libterm.com
libterm.com