Polymer materials are essential in modern manufacturing due to their versatility, durability, and lightweight properties. Their applications span industries from packaging and automotive to healthcare and electronics, making them integral to everyday products. Discover how polymers impact your daily life and the future of technology by reading the full article.
Table of Comparison
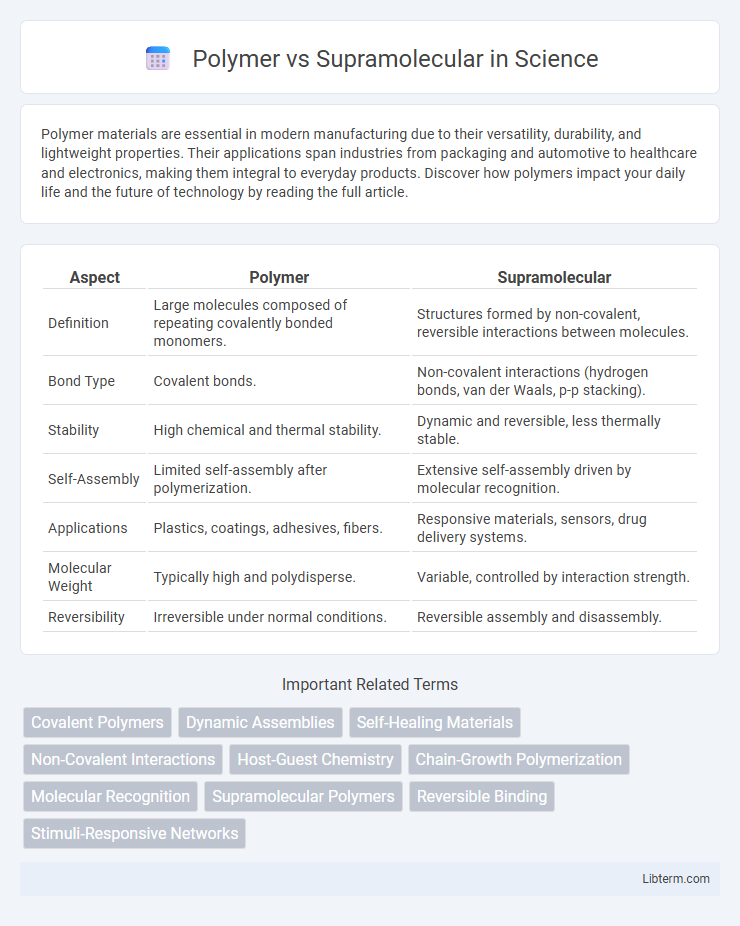
| Aspect | Polymer | Supramolecular |
|---|---|---|
| Definition | Large molecules composed of repeating covalently bonded monomers. | Structures formed by non-covalent, reversible interactions between molecules. |
| Bond Type | Covalent bonds. | Non-covalent interactions (hydrogen bonds, van der Waals, p-p stacking). |
| Stability | High chemical and thermal stability. | Dynamic and reversible, less thermally stable. |
| Self-Assembly | Limited self-assembly after polymerization. | Extensive self-assembly driven by molecular recognition. |
| Applications | Plastics, coatings, adhesives, fibers. | Responsive materials, sensors, drug delivery systems. |
| Molecular Weight | Typically high and polydisperse. | Variable, controlled by interaction strength. |
| Reversibility | Irreversible under normal conditions. | Reversible assembly and disassembly. |
Introduction to Polymers and Supramolecular Chemistry
Polymers are large macromolecules composed of repeating structural units called monomers, linked through covalent bonds, and they serve as the foundation for plastics, fibers, and many synthetic materials. Supramolecular chemistry explores non-covalent interactions such as hydrogen bonding, metal coordination, and van der Waals forces to assemble discrete molecular entities into organized structures with dynamic and reversible properties. Understanding the fundamental differences in bonding and assembly between polymers and supramolecular systems enables the design of advanced materials with tailored mechanical, chemical, and functional characteristics.
Defining Polymers: Structure and Properties
Polymers consist of long chains of repeating covalently bonded monomers, providing structural stability and mechanical strength, while supramolecular assemblies rely on reversible, non-covalent interactions such as hydrogen bonding, van der Waals forces, and metal coordination, enabling dynamic and stimuli-responsive properties. The molecular weight distribution and chain architecture in polymers determine their thermal and viscoelastic behavior, contrasting with supramolecular systems that exhibit self-healing and adaptability due to their transient bond networks. Understanding these fundamental differences is crucial in tailoring materials for specific applications like drug delivery, smart coatings, and flexible electronics.
Supramolecular Systems: Key Concepts and Components
Supramolecular systems consist of molecular entities held together by non-covalent interactions such as hydrogen bonding, van der Waals forces, and electrostatic interactions, enabling dynamic and reversible assemblies. Unlike polymers, which are covalently bonded macromolecules, supramolecular structures exhibit stimuli-responsive behavior and can self-assemble, leading to applications in drug delivery, sensing, and nanotechnology. Key components include host-guest complexes, molecular recognition motifs, and adaptive frameworks critical for designing functional materials.
Synthesis Methods: Polymerization vs. Self-Assembly
Polymer synthesis primarily involves polymerization techniques such as addition, condensation, and copolymerization, enabling the formation of long-chain macromolecules with precise control over molecular weight and architecture. Supramolecular synthesis relies on self-assembly processes driven by non-covalent interactions, including hydrogen bonding, p-p stacking, and metal coordination, allowing dynamic and reversible formation of complex structures. Understanding these distinct synthesis methods is crucial for designing materials with tailored properties for applications in nanotechnology, drug delivery, and smart materials.
Structural Differences: Covalent vs. Non-covalent Bonds
Polymers consist of long chains of monomers linked by strong covalent bonds, providing structural stability and durability. Supramolecular assemblies rely on non-covalent interactions such as hydrogen bonding, van der Waals forces, and electrostatic interactions, enabling reversible and dynamic structure formation. These fundamental bonding differences result in distinct mechanical properties and functional responsiveness for polymers versus supramolecular materials.
Functional Diversity and Applications
Polymers exhibit extensive functional diversity through their covalently bonded repeating units, enabling tailored mechanical, thermal, and chemical properties for applications in packaging, automotive parts, and electronics. Supramolecular materials leverage non-covalent interactions such as hydrogen bonding and metal coordination, offering reversible and stimuli-responsive functionalities ideal for drug delivery, self-healing materials, and sensors. The dynamic nature of supramolecular assemblies contrasts with the structural stability of polymers, expanding the scope of applications where adaptability and molecular recognition are critical.
Mechanical and Thermal Properties Comparison
Polymers typically exhibit high mechanical strength and good thermal stability due to covalent bonding within their molecular chains, making them suitable for structural applications. Supramolecular materials rely on non-covalent interactions such as hydrogen bonding and p-p stacking, resulting in tunable mechanical properties but generally lower thermal resistance compared to polymers. Compared to polymers, supramolecular assemblies offer reversible mechanical behavior and enhanced self-healing capabilities but often sacrifice the thermal stability required for high-temperature applications.
Responsiveness and Stimuli-Adaptivity
Polymers exhibit responsiveness primarily through covalent bond changes or physical rearrangements triggered by stimuli such as temperature, pH, or light, enabling controlled drug release and shape memory behavior. Supramolecular systems rely on reversible non-covalent interactions like hydrogen bonding, metal coordination, or p-p stacking, offering rapid, reversible stimuli-adaptivity and self-healing properties. The dynamic and tunable nature of supramolecular assemblies provides enhanced sensitivity and selectivity compared to conventional polymers in applications such as sensors and smart materials.
Environmental Impact and Sustainability
Polymers often involve synthetic materials derived from petrochemicals with significant environmental footprints due to their non-biodegradable nature and energy-intensive production processes. Supramolecular materials, designed through reversible and non-covalent interactions, promote sustainability by enabling recyclability, self-healing properties, and reduced waste generation. Advances in supramolecular chemistry focus on creating eco-friendly, biodegradable alternatives that minimize pollution and support circular economy initiatives.
Future Perspectives: Trends in Polymer and Supramolecular Research
Emerging trends in polymer research emphasize the development of sustainable, biodegradable materials with enhanced mechanical properties and stimuli-responsive behaviors. Supramolecular chemistry is advancing toward dynamic, self-healing systems and adaptive architectures driven by non-covalent interactions for applications in drug delivery and smart materials. Future perspectives underscore the integration of polymer and supramolecular methodologies to create multifunctional materials with tunable properties for environmental, biomedical, and electronic innovations.
Polymer Infographic
 libterm.com
libterm.com