Unsaturated compounds contain one or more double or triple bonds between carbon atoms, allowing them to react differently than saturated compounds that have only single bonds. These molecules play a crucial role in various biological and industrial processes, such as the formation of essential fatty acids and the production of polymers. Explore the full article to understand how unsaturation impacts your daily life and industry applications.
Table of Comparison
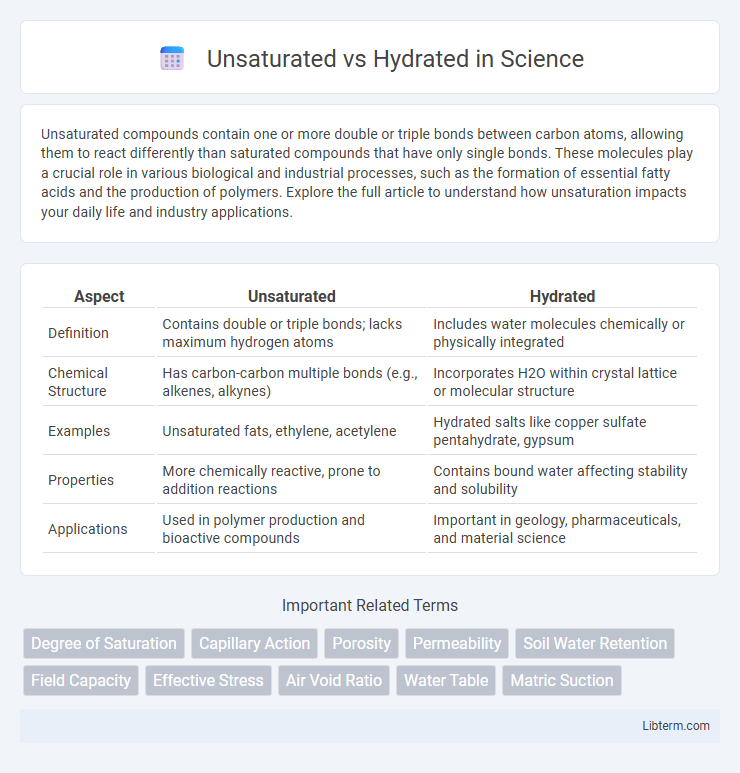
| Aspect | Unsaturated | Hydrated |
|---|---|---|
| Definition | Contains double or triple bonds; lacks maximum hydrogen atoms | Includes water molecules chemically or physically integrated |
| Chemical Structure | Has carbon-carbon multiple bonds (e.g., alkenes, alkynes) | Incorporates H2O within crystal lattice or molecular structure |
| Examples | Unsaturated fats, ethylene, acetylene | Hydrated salts like copper sulfate pentahydrate, gypsum |
| Properties | More chemically reactive, prone to addition reactions | Contains bound water affecting stability and solubility |
| Applications | Used in polymer production and bioactive compounds | Important in geology, pharmaceuticals, and material science |
Understanding Unsaturated and Hydrated States
Unsaturated refers to a chemical state where molecules contain double or triple bonds, allowing the addition of more atoms without breaking existing bonds, commonly seen in unsaturated fats and hydrocarbons. Hydrated states involve molecules or compounds chemically combined with water molecules, often forming hydrates in minerals or crystalline substances. Understanding these states is crucial in fields like chemistry and nutrition, as unsaturated compounds exhibit different reactivity and health properties, while hydration affects substance stability and biological functions.
Key Definitions: Unsaturated vs Hydrated
Unsaturated refers to a solution or compound that contains less solute than the maximum amount that can be dissolved at a given temperature, indicating it has the capacity to dissolve more solute. Hydrated describes a compound, typically a salt, that includes water molecules integrated within its crystal structure, forming a hydrate. The distinction between unsaturated and hydrated lies in unsaturated relating to solute concentration in a solution, while hydrated pertains to the presence of water molecules chemically bound in a solid compound.
Chemical Structure Differences
Unsaturated compounds contain one or more double or triple bonds between carbon atoms, creating regions of higher reactivity and altering molecular geometry. Hydrated compounds incorporate water molecules either through chemical bonding or physical adsorption, resulting in the presence of hydroxyl groups or water of crystallization within the structure. The key chemical structure difference lies in unsaturation involving carbon-carbon multiple bonds, whereas hydration involves the integration of water molecules into the molecular framework.
Physical Properties Comparison
Unsaturated compounds typically exhibit lower melting and boiling points due to the presence of double or triple bonds, which create kinks in their molecular structure, reducing packing efficiency. Hydrated compounds contain water molecules integrated into their crystal lattice, increasing their mass and often resulting in higher melting points and altered solubility compared to their unsaturated counterparts. The presence or absence of hydration significantly affects physical properties like density, heat capacity, and stability, making these distinctions crucial in materials science and chemical synthesis.
Role in Industrial Applications
Unsaturated compounds play a crucial role in industrial applications such as the production of polymers, synthetic rubbers, and resins due to their reactive double or triple bonds that enable polymerization and chemical modifications. Hydrated compounds are essential in industries like pharmaceuticals, construction, and food processing, where water molecules integrated into their structure affect solubility, stability, and reaction mechanisms. Understanding the distinct chemical behaviors of unsaturated versus hydrated substances allows engineers to optimize manufacturing processes, enhance product performance, and improve material properties.
Environmental Impact and Safety
Unsaturated compounds typically have higher reactivity and can release volatile organic compounds (VOCs) that contribute to air pollution and ozone layer depletion, posing environmental hazards. Hydrated substances generally exhibit lower volatility and reduced flammability, enhancing safety in handling and storage while minimizing environmental contamination risks. Proper management of unsaturated chemicals is essential to mitigate toxic emissions, whereas hydrated forms often offer safer alternatives with less ecological impact.
Effects on Material Durability
Unsaturated materials tend to exhibit higher mechanical strength and resistance to deformation due to lower water content limiting molecular mobility. Hydrated materials absorb moisture, which can induce swelling, reduce tensile strength, and accelerate chemical degradation processes such as hydrolysis. These effects collectively decrease the long-term durability and structural integrity of materials exposed to humid or aqueous environments.
Common Examples in Everyday Life
Unsaturated fats are commonly found in olive oil, avocados, and nuts, promoting heart health by reducing bad cholesterol levels. Hydrated substances, such as hydrated salts like gypsum in drywall or hydrated carbohydrates in fruits and vegetables, play a crucial role in everyday hydration and structural functions. Understanding the distinction helps in making healthier dietary choices and recognizing the importance of water content in various materials used daily.
Importance in Biological Systems
Unsaturated compounds play a crucial role in biological membranes by maintaining fluidity and enabling proper function of cellular processes, while hydrated molecules are essential for biochemical reactions and maintaining cellular homeostasis through water-mediated interactions. The balance between unsaturated lipids and hydration levels affects membrane permeability, enzyme activity, and signal transduction pathways critical to life. Understanding these factors helps in studying metabolic health, cellular communication, and the structural integrity of biological systems.
Choosing Between Unsaturated and Hydrated Products
Choosing between unsaturated and hydrated products depends on the desired texture and moisture content in food or cosmetic applications. Unsaturated products, rich in double bonds, offer better fluidity and ease of absorption but are prone to oxidation, affecting shelf life. Hydrated products contain water molecules that enhance softness and improve hydration but may require preservatives to prevent microbial growth.
Unsaturated Infographic
 libterm.com
libterm.com