Necroptosis is a regulated form of cell death that combines features of both necrosis and apoptosis, playing a crucial role in inflammation and immune response. This programmed mechanism involves key proteins like RIPK1 and RIPK3, leading to membrane rupture and the release of cellular contents that trigger inflammation. Discover how necroptosis impacts disease progression and therapeutic strategies by reading the rest of this article.
Table of Comparison
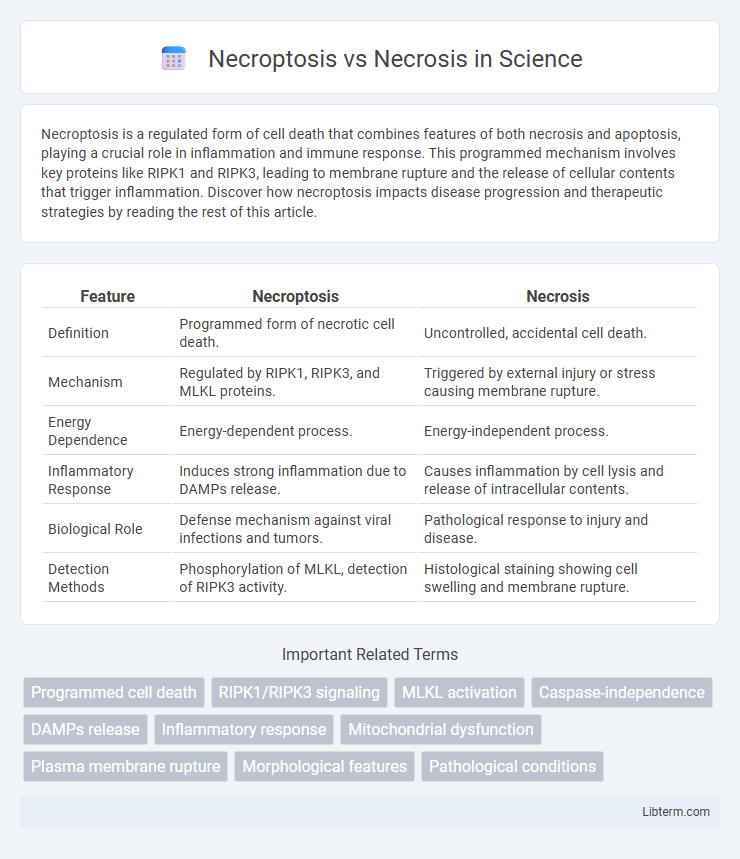
| Feature | Necroptosis | Necrosis |
|---|---|---|
| Definition | Programmed form of necrotic cell death. | Uncontrolled, accidental cell death. |
| Mechanism | Regulated by RIPK1, RIPK3, and MLKL proteins. | Triggered by external injury or stress causing membrane rupture. |
| Energy Dependence | Energy-dependent process. | Energy-independent process. |
| Inflammatory Response | Induces strong inflammation due to DAMPs release. | Causes inflammation by cell lysis and release of intracellular contents. |
| Biological Role | Defense mechanism against viral infections and tumors. | Pathological response to injury and disease. |
| Detection Methods | Phosphorylation of MLKL, detection of RIPK3 activity. | Histological staining showing cell swelling and membrane rupture. |
Introduction to Cell Death Mechanisms
Necroptosis is a programmed form of necrotic cell death regulated by receptor-interacting protein kinases RIPK1 and RIPK3, leading to membrane rupture and inflammation. Necrosis, in contrast, is an uncontrolled process caused by acute cellular injury, resulting in cell swelling and plasma membrane disruption without specific signaling. Understanding these distinct cell death pathways is crucial for therapeutic targeting in diseases involving inflammation and tissue damage.
Defining Necroptosis: Programmed Necrosis
Necroptosis is a form of programmed necrosis characterized by regulated cellular death mechanisms involving receptor-interacting protein kinases RIPK1 and RIPK3, leading to membrane rupture and inflammation. Unlike necrosis, which is uncontrolled and results from acute injury causing cell lysis, necroptosis follows a defined signaling pathway triggered by stimuli such as TNF-a and viral infections. This controlled process serves as a backup cell death pathway when apoptosis is inhibited, playing a crucial role in immune response and disease pathology.
What is Necrosis?
Necrosis is a form of uncontrolled cell death resulting from acute cellular injury, leading to the rupture of the plasma membrane and the release of intracellular contents that trigger inflammation. It typically occurs due to factors such as ischemia, toxins, or trauma, causing tissue damage and loss of function. Unlike necroptosis, which is a regulated programmed process, necrosis is passive and unregulated, contributing to pathological conditions.
Molecular Pathways: Necroptosis vs Necrosis
Necroptosis is a programmed necrotic cell death regulated by RIPK1, RIPK3, and MLKL proteins, leading to plasma membrane rupture and inflammatory responses. In contrast, necrosis is a passive, unregulated process typically caused by external injury, resulting in uncontrolled cell swelling and membrane breakdown without specific molecular signaling. The necroptotic pathway involves the formation of the necrosome complex, whereas necrosis lacks defined molecular intermediates.
Key Triggers and Stimuli
Necroptosis is primarily triggered by death receptors such as TNF receptor 1 (TNFR1) and pattern recognition receptors upon pathogen detection, involving the activation of receptor-interacting protein kinases RIPK1 and RIPK3. Necrosis is typically initiated by overwhelming cellular damage from physical injury, toxins, or ischemic conditions leading to uncontrolled cell swelling and plasma membrane rupture. While necroptosis is a regulated form of programmed necrosis driven by specific signaling pathways, necrosis results from incidental stress without programmed control.
Morphological Differences Explained
Necroptosis exhibits distinct morphological features such as cell swelling, plasma membrane rupture, and organelle dilation, which are tightly regulated by receptor-interacting protein kinases RIPK1 and RIPK3, in contrast to necrosis characterized by uncontrolled cellular disintegration and inflammation resulting from external injury. Unlike necrosis, necroptosis maintains an organized signaling pathway leading to a programmed form of cell death, with preserved nuclear integrity until late stages. Understanding the ultrastructural differences, including membrane permeability and mitochondrial changes, is crucial for distinguishing the regulated necroptotic process from passive necrotic damage.
Role in Disease and Pathophysiology
Necroptosis is a programmed form of necrotic cell death mediated by receptor-interacting protein kinase 1 and 3 (RIPK1/RIPK3) and mixed lineage kinase domain-like protein (MLKL), playing a critical role in inflammatory diseases and tissue damage. Unlike necrosis, which occurs passively due to acute injury or trauma causing uncontrolled cell lysis and inflammation, necroptosis actively promotes inflammation through the release of damage-associated molecular patterns (DAMPs). Dysregulated necroptosis contributes to the pathophysiology of neurodegenerative disorders, ischemic injury, and autoimmune diseases by exacerbating cell death and inflammatory responses.
Diagnostic Biomarkers and Detection
Necroptosis and necrosis exhibit distinct diagnostic biomarkers vital for differential detection in clinical pathology. Necroptosis is characterized by the activation of receptor-interacting protein kinases RIPK1 and RIPK3, along with phosphorylated mixed lineage kinase domain-like protein (p-MLKL), detectable through immunohistochemistry and Western blotting. In contrast, necrosis is identified by cellular swelling, loss of membrane integrity, and elevated levels of lactate dehydrogenase (LDH) released into the extracellular environment, measurable through biochemical assays and histological staining.
Therapeutic Implications and Drug Targets
Necroptosis, a regulated form of necrotic cell death mediated by receptor-interacting protein kinases RIPK1 and RIPK3, presents distinct therapeutic targets compared to unregulated necrosis, which lacks signaling specificity. Inhibitors targeting key necroptotic proteins such as MLKL and RIPK3 hold promise for treating inflammatory and degenerative diseases by preventing excessive cell death and tissue damage. Understanding the molecular differences between necroptosis and necrosis enables drug development focused on modulating programmed necrosis pathways, offering novel strategies for conditions like ischemia-reperfusion injury and neurodegeneration.
Future Perspectives in Cell Death Research
Future perspectives in cell death research emphasize the distinction between necroptosis and necrosis by exploring novel molecular targets for therapeutic intervention in inflammatory and degenerative diseases. Advancements in understanding necroptosis signaling pathways, such as RIPK1, RIPK3, and MLKL, pave the way for precision medicine approaches that selectively inhibit programmed necrotic cell death without compromising apoptosis. Emerging technologies like single-cell RNA sequencing and high-throughput screening will accelerate the discovery of biomarkers and drug candidates that modulate necroptosis, enhancing treatment efficacy and minimizing off-target effects.
Necroptosis Infographic
 libterm.com
libterm.com