Geometric isomers are compounds that have the same molecular formula but differ in the spatial arrangement of atoms around a double bond or ring structure. This difference significantly influences their physical and chemical properties, impacting everything from boiling points to biological activity. Explore the rest of this article to understand how geometric isomers affect molecular behavior and why they are crucial in chemistry and pharmacology.
Table of Comparison
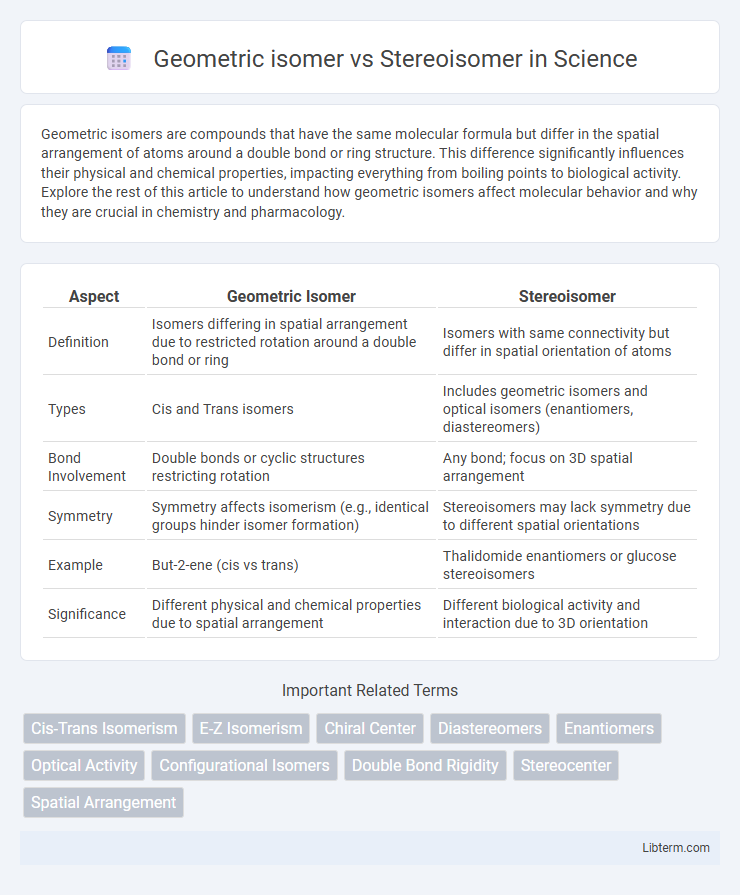
| Aspect | Geometric Isomer | Stereoisomer |
|---|---|---|
| Definition | Isomers differing in spatial arrangement due to restricted rotation around a double bond or ring | Isomers with same connectivity but differ in spatial orientation of atoms |
| Types | Cis and Trans isomers | Includes geometric isomers and optical isomers (enantiomers, diastereomers) |
| Bond Involvement | Double bonds or cyclic structures restricting rotation | Any bond; focus on 3D spatial arrangement |
| Symmetry | Symmetry affects isomerism (e.g., identical groups hinder isomer formation) | Stereoisomers may lack symmetry due to different spatial orientations |
| Example | But-2-ene (cis vs trans) | Thalidomide enantiomers or glucose stereoisomers |
| Significance | Different physical and chemical properties due to spatial arrangement | Different biological activity and interaction due to 3D orientation |
Introduction to Isomerism
Isomerism refers to the phenomenon where compounds share the same molecular formula but differ in structural arrangement or spatial configuration. Geometric isomers are a type of stereoisomer characterized by different spatial orientations around a double bond or a ring structure, resulting in cis/trans configurations. Stereoisomers encompass a broader category including all isomers with identical bonds but differing in three-dimensional arrangements, such as geometric isomers and enantiomers.
Defining Stereoisomers
Stereoisomers are molecules with the same molecular formula and sequence of bonded atoms but differ in the three-dimensional orientations of their atoms in space. Geometric isomers are a subtype of stereoisomers characterized by restricted rotation around a double bond or ring structure, resulting in cis and trans configurations. Defining stereoisomers involves understanding their spatial arrangements, including optical isomers and geometric isomers, which influence physical and chemical properties without altering connectivity.
What are Geometric Isomers?
Geometric isomers are a specific type of stereoisomers characterized by the spatial arrangement of atoms or groups around a double bond or a ring structure, leading to distinct physical and chemical properties despite having the same molecular formula. These isomers arise due to restricted rotation around the bond, resulting in cis (same side) and trans (opposite side) configurations. Unlike other stereoisomers, geometric isomers differ primarily in their spatial orientation rather than connectivity, affecting boiling points, solubility, and reactivity.
Types of Stereoisomers: Geometric vs Optical
Stereoisomers are compounds with the same molecular formula and connectivity but differ in spatial arrangement, categorized into geometric and optical isomers. Geometric isomers, also called cis-trans isomers, occur due to restricted rotation around double bonds or ring structures, influencing physical properties like boiling points and solubility. Optical isomers, or enantiomers, arise from chiral centers and exhibit non-superimposable mirror images, crucial in pharmacology due to their different interactions with biological systems.
Structural Characteristics of Geometric Isomers
Geometric isomers, a subset of stereoisomers, differ specifically in the spatial arrangement of groups around a rigid structure such as a double bond or a ring system, leading to distinct cis and trans configurations. The restricted rotation around the double bond or ring is a key structural characteristic that prevents these isomers from interconverting easily, resulting in unique physical and chemical properties. This rigidity in geometric isomers contrasts with other stereoisomers, which may differ in spatial arrangement without such rotational constraints.
Spatial Arrangement in Stereoisomers
Stereoisomers differ in the spatial arrangement of atoms without changing the molecular formula, encompassing both geometric isomers and optical isomers. Geometric isomers specifically involve variations around a double bond or a ring structure, where the relative positions of substituents differ as cis or trans configurations. The spatial arrangement in stereoisomers is critical because it influences physical and chemical properties, such as polarity, reactivity, and interaction with biological molecules.
Geometric Isomerism: Cis-Trans and E-Z Notation
Geometric isomerism, a subtype of stereoisomerism, refers to compounds with the same molecular formula but different spatial arrangements around a double bond or ring structure. Cis-trans isomerism occurs when similar groups are positioned adjacent (cis) or opposite (trans) each other across a double bond, influencing physical and chemical properties. The E-Z notation expands on this by assigning priority to substituents based on atomic number, with 'E' indicating opposite sides and 'Z' indicating the same side, providing a more precise and universal system for describing stereochemistry.
Differences Between Geometric and Other Stereoisomers
Geometric isomers, a subset of stereoisomers, differ primarily in the spatial arrangement of groups around a double bond or ring structure, where restricted rotation causes distinct cis and trans forms. Other stereoisomers, such as enantiomers and diastereomers, involve variations in the 3D orientation of atoms around chiral centers, rather than restricted bonds. The key difference lies in geometric isomers exhibiting isomerism due to restricted rotation leading to different spatial configurations, whereas other stereoisomers arise from different spatial arrangements of atoms without bond rotation constraints.
Chemical and Physical Properties Comparison
Geometric isomers, a subset of stereoisomers, differ in the spatial arrangement of groups around a double bond or ring structure, which significantly affects their chemical reactivity and physical properties such as boiling points and solubility. Stereoisomers, including enantiomers and diastereomers, share the same molecular formula and connectivity but vary in three-dimensional orientation, influencing optical activity and interaction with chiral environments. The distinct physical properties of geometric isomers, such as cis isomers having higher polarity and boiling points compared to their trans counterparts, contrast with stereoisomers, where differences in optical rotation and chiral recognition are more pronounced.
Importance and Applications of Geometric and Stereoisomers
Geometric and stereoisomers play critical roles in pharmaceuticals, where their distinct spatial arrangements can drastically affect drug efficacy and safety. Geometric isomers, such as cis-trans forms, are essential in designing molecules with specific physical properties and reactivity, impacting materials science and agrochemicals. Stereoisomers, including enantiomers and diastereomers, are crucial in chiral synthesis, influencing biochemical interactions and the development of enantioselective catalysts.
Geometric isomer Infographic
 libterm.com
libterm.com