Chaperone-mediated autophagy (CMA) is a selective cellular process that degrades damaged or dysfunctional proteins by targeting them to lysosomes for breakdown. This pathway plays a crucial role in maintaining protein quality control and cellular homeostasis, impacting aging and disease progression. Discover how your cells harness CMA to protect against stress and maintain optimal function in the detailed overview below.
Table of Comparison
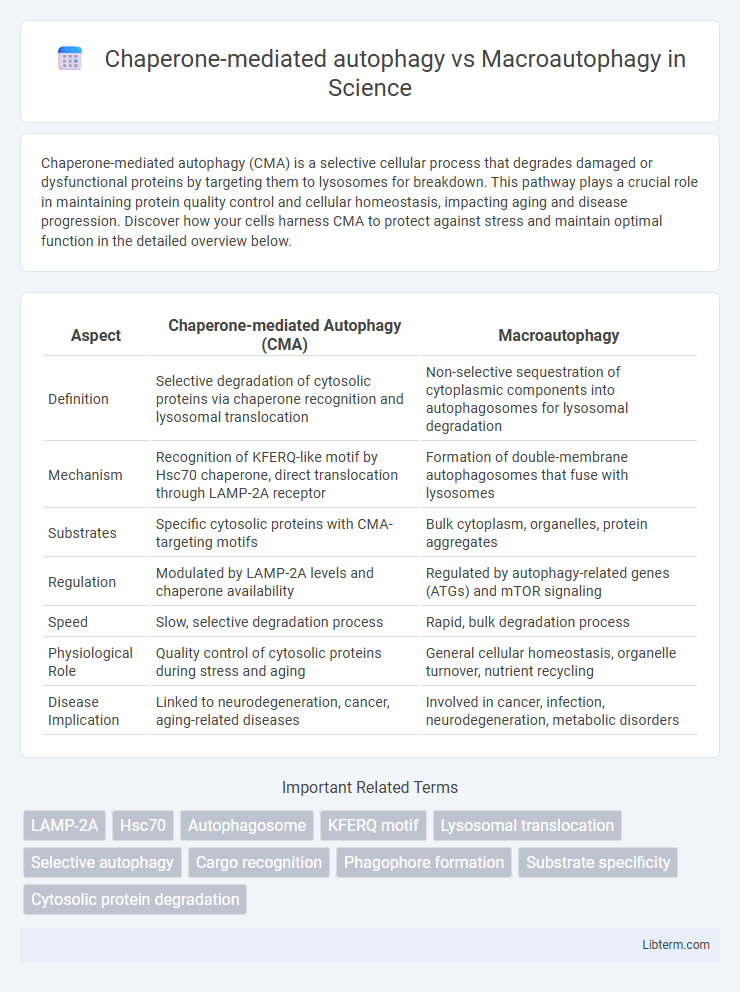
| Aspect | Chaperone-mediated Autophagy (CMA) | Macroautophagy |
|---|---|---|
| Definition | Selective degradation of cytosolic proteins via chaperone recognition and lysosomal translocation | Non-selective sequestration of cytoplasmic components into autophagosomes for lysosomal degradation |
| Mechanism | Recognition of KFERQ-like motif by Hsc70 chaperone, direct translocation through LAMP-2A receptor | Formation of double-membrane autophagosomes that fuse with lysosomes |
| Substrates | Specific cytosolic proteins with CMA-targeting motifs | Bulk cytoplasm, organelles, protein aggregates |
| Regulation | Modulated by LAMP-2A levels and chaperone availability | Regulated by autophagy-related genes (ATGs) and mTOR signaling |
| Speed | Slow, selective degradation process | Rapid, bulk degradation process |
| Physiological Role | Quality control of cytosolic proteins during stress and aging | General cellular homeostasis, organelle turnover, nutrient recycling |
| Disease Implication | Linked to neurodegeneration, cancer, aging-related diseases | Involved in cancer, infection, neurodegeneration, metabolic disorders |
Introduction to Cellular Autophagy Pathways
Chaperone-mediated autophagy (CMA) selectively degrades cytosolic proteins by recognizing KFERQ-like motifs, targeting them to lysosomes via LAMP-2A receptors, whereas macroautophagy engulfs bulk cytoplasmic components, including organelles, within double-membrane autophagosomes that fuse with lysosomes for degradation. Both pathways are critical for maintaining cellular homeostasis, stress response, and protein quality control, but differ in mechanisms and substrate specificity. Dysregulation of CMA and macroautophagy is implicated in neurodegenerative diseases, cancer, and aging, highlighting their distinct yet complementary roles in cellular autophagy.
Overview of Chaperone-Mediated Autophagy (CMA)
Chaperone-mediated autophagy (CMA) selectively targets cytosolic proteins containing a KFERQ-like motif for lysosomal degradation via direct translocation across the lysosomal membrane facilitated by the LAMP-2A receptor. Unlike macroautophagy, which engulfs bulk cytoplasmic portions into autophagosomes, CMA operates without vesicle formation, enabling precise regulation of protein quality and cellular homeostasis. CMA plays a specialized role in stress response and metabolic adaptation by selectively degrading damaged or misfolded proteins, thereby maintaining proteostasis and preventing cellular dysfunction.
Defining Macroautophagy: Mechanism and Function
Macroautophagy is a cellular degradation process involving the formation of double-membrane vesicles called autophagosomes that engulf cytoplasmic components, including damaged organelles and protein aggregates, for lysosomal degradation. This mechanism plays a crucial role in maintaining cellular homeostasis by recycling nutrients and removing toxic materials during stress or nutrient deprivation. Unlike chaperone-mediated autophagy, which selectively degrades specific proteins through lysosomal translocation, macroautophagy handles bulk degradation of cytoplasmic content in a non-selective or selective manner.
Key Molecular Components: CMA vs Macroautophagy
Chaperone-mediated autophagy (CMA) relies on the cytosolic chaperone Hsc70 and the lysosomal receptor LAMP-2A to selectively translocate targeted proteins containing a KFERQ-like motif into the lysosome. Macroautophagy depends on core autophagy-related (ATG) proteins, including the ULK1 complex for initiation, the Beclin-1-VPS34 complex for nucleation, and LC3-II conjugation for autophagosome membrane elongation and cargo sequestration. Distinct molecular mechanisms and protein machinery differentiate CMA's substrate specificity from the bulk degradation process orchestrated by macroautophagy.
Substrate Recognition and Selectivity
Chaperone-mediated autophagy (CMA) selectively targets soluble cytosolic proteins containing a KFERQ-like motif recognized by the cytosolic chaperone Hsc70, which facilitates direct translocation into lysosomes via LAMP-2A receptors. Macroautophagy engulfs diverse cytoplasmic cargo, including proteins, organelles, and aggregates, through the formation of double-membrane autophagosomes guided by ubiquitin signals and selective adaptor proteins like p62. CMA exhibits high substrate selectivity based on motif recognition, while macroautophagy provides broader, less selective degradation responses to cellular stress.
Lysosomal Uptake Mechanisms Compared
Chaperone-mediated autophagy (CMA) selectively targets cytosolic proteins containing a KFERQ-like motif for lysosomal degradation via direct translocation through the lysosomal-associated membrane protein 2A (LAMP-2A) receptor. Macroautophagy engulfs bulk cytoplasmic content, including organelles and proteins, within double-membrane autophagosomes that fuse with lysosomes for degradation. Unlike macroautophagy's vesicle-mediated delivery, CMA relies on receptor-mediated translocation, enabling selective protein degradation without vesicle formation.
Regulation Pathways: CMA and Macroautophagy
Chaperone-mediated autophagy (CMA) is regulated primarily through the lysosomal-associated membrane protein 2A (LAMP-2A) receptor, whose levels and multimerization control substrate translocation into lysosomes. Macroautophagy regulation involves key signaling pathways including the mechanistic target of rapamycin complex 1 (mTORC1), which inhibits autophagy initiation, and AMP-activated protein kinase (AMPK), which activates the ULK1 complex to promote autophagosome formation. Both pathways integrate cellular stress signals, but CMA selectively degrades soluble cytosolic proteins, whereas macroautophagy targets larger substrates including organelles via autophagosome-lysosome fusion.
Physiological Roles and Cellular Impact
Chaperone-mediated autophagy (CMA) selectively degrades cytosolic proteins with a KFERQ-like motif, playing a crucial role in protein quality control, energy homeostasis, and stress response by directly translocating substrates into lysosomes. Macroautophagy engulfs bulk cytoplasmic components, including damaged organelles and protein aggregates, via autophagosomes, contributing to cellular remodeling, metabolic adaptation, and organelle turnover. Both pathways maintain cellular homeostasis, but CMA provides specificity in protein degradation, while macroautophagy offers broad cytoprotective functions during nutrient deprivation and cellular stress.
Implications in Disease: CMA vs Macroautophagy
Chaperone-mediated autophagy (CMA) selectively degrades cytosolic proteins with KFERQ-like motifs, playing a critical role in neurodegenerative diseases such as Parkinson's and Alzheimer's by preventing toxic protein aggregation. Macroautophagy engulfs bulk cytoplasmic content, including damaged organelles and protein aggregates, and its impairment is implicated in cancer, infectious diseases, and metabolic disorders. Differences in substrate specificity and degradation pathways between CMA and macroautophagy influence disease progression and therapeutic targeting strategies.
Therapeutic Potential and Future Perspectives
Chaperone-mediated autophagy (CMA) selectively degrades cytosolic proteins via lysosomal translocation, offering precise therapeutic targets for neurodegenerative diseases and cancer by modulating protein quality control. Macroautophagy engulfs bulk cellular components, enabling widespread clearance of damaged organelles and aggregates, making it a promising strategy for metabolic disorders and aging-related pathologies. Future perspectives emphasize enhancing CMA specificity and macroautophagy efficiency through pharmacological agents and gene editing tools to optimize disease treatment outcomes.
Chaperone-mediated autophagy Infographic
 libterm.com
libterm.com