Conformers are different spatial arrangements of a molecule that can be interconverted by rotation around single bonds without breaking covalent bonds. Understanding conformational analysis is crucial for predicting the physical and chemical properties of compounds, including reactivity and stability. Explore the rest of the article to learn how conformers influence molecular behavior and your applications in chemistry.
Table of Comparison
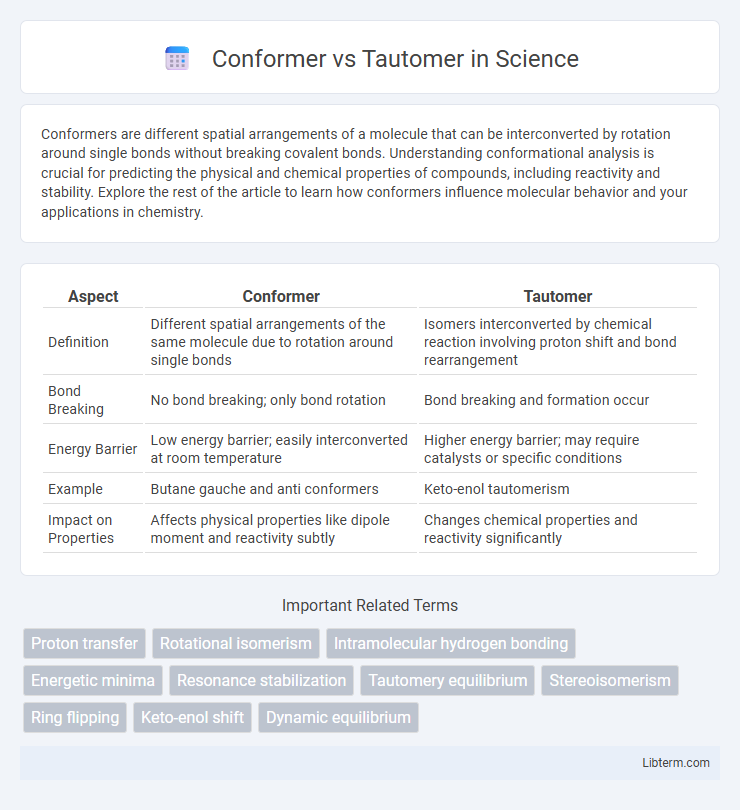
| Aspect | Conformer | Tautomer |
|---|---|---|
| Definition | Different spatial arrangements of the same molecule due to rotation around single bonds | Isomers interconverted by chemical reaction involving proton shift and bond rearrangement |
| Bond Breaking | No bond breaking; only bond rotation | Bond breaking and formation occur |
| Energy Barrier | Low energy barrier; easily interconverted at room temperature | Higher energy barrier; may require catalysts or specific conditions |
| Example | Butane gauche and anti conformers | Keto-enol tautomerism |
| Impact on Properties | Affects physical properties like dipole moment and reactivity subtly | Changes chemical properties and reactivity significantly |
Introduction to Conformers and Tautomers
Conformers are different spatial arrangements of a molecule that can be interconverted by rotation around single bonds without breaking any bonds, influencing properties such as reactivity and stability. Tautomers are isomers of a compound that differ by the position of a proton and a double bond, typically existing in dynamic equilibrium, with keto-enol tautomerism being a common example. Understanding the distinctions between conformers and tautomers is crucial in fields like drug design and chemical synthesis for predicting molecular behavior.
Definition of Conformer
A conformer is a specific spatial arrangement of atoms in a molecule that can be interconverted by rotation around single bonds without breaking any chemical bonds. Unlike tautomers, which are isomers differing by the position of a proton and a double bond, conformers exist in dynamic equilibrium due to internal rotations. Conformational analysis is critical in understanding molecular behavior, stability, and reactivity in organic chemistry and biochemistry.
Definition of Tautomer
Tautomers are isomers of a compound that differ only in the position of protons and electrons, typically involving the migration of a hydrogen atom and a switch between single and double bonds, such as keto-enol tautomerism. Conformers, in contrast, are different spatial arrangements of the same molecule that can be interconverted by rotation around single bonds without breaking any bonds. Understanding tautomerism is crucial in chemistry and biochemistry as it affects molecular stability, reactivity, and biological activity.
Key Differences Between Conformers and Tautomers
Conformers are different spatial arrangements of a molecule generated by rotation around single bonds without breaking any bonds, whereas tautomers are isomers that differ by the position of a proton and a double bond involving bond breaking and formation. Conformers interconvert rapidly at room temperature through bond rotation, while tautomers interconvert through chemical reactions involving proton transfer. The key difference lies in conformational flexibility versus tautomeric chemical equilibrium, impacting molecular properties and reactivity distinctly.
Structural Features of Conformers
Conformers are distinct spatial arrangements of atoms resulting from rotation around single (sigma) bonds without breaking covalent bonds, characterized by varying dihedral angles that define their three-dimensional shapes. These structural features influence molecular flexibility and impact physical properties like boiling points and reaction rates. Unlike tautomers, conformers do not involve changes in connectivity or proton transfer, making their interconversion a simple rotational isomerism.
Structural Features of Tautomers
Tautomers exhibit structural features characterized by the migration of a proton and a shift in the position of a double bond, resulting in distinct isomers that differ in connectivity rather than rotation around single bonds. Unlike conformers, which are interconverted solely by rotations around sigma bonds, tautomers involve the making and breaking of covalent bonds, typically seen in keto-enol or imine-enamine pairs. This structural rearrangement leads to different electronic distributions and chemical properties, critical in biochemical processes and pharmaceutical applications.
Mechanisms of Interconversion
Conformers interconvert through rotations about single sigma bonds, enabling molecules to adopt different spatial arrangements without breaking covalent bonds. Tautomers interconvert via proton transfer accompanied by the relocation of double bonds, typically involving keto-enol or imine-enamine equilibria. The energy barriers for conformational changes are generally lower than those for tautomerization, reflecting distinct mechanistic pathways governed by bond rotation versus proton migration.
Importance in Organic Chemistry
Conformers represent different spatial arrangements of a molecule that can interconvert through rotation around single bonds and significantly influence the molecule's steric interactions and reactivity profiles. Tautomers are isomers that readily interconvert by the transfer of a proton and a shift in bonding electrons, playing a crucial role in mechanisms such as keto-enol tautomerism affecting acidity and nucleophilicity. Understanding the dynamic equilibrium between conformers and tautomers is essential for predicting reaction pathways, stability, and the behavior of organic compounds in synthesis and biological systems.
Methods of Identification and Analysis
Conformers are identified primarily through spectroscopic techniques such as nuclear magnetic resonance (NMR) and infrared (IR) spectroscopy, which detect differences in spatial arrangement without bond rearrangement. Tautomers are distinguished using methods that reveal positional changes of atoms or bonds, including UV-visible spectroscopy and mass spectrometry, alongside NMR to monitor proton shifts. Computational chemistry tools, including density functional theory (DFT), complement experimental techniques by predicting energy barriers and stability between conformers and tautomers for accurate analysis.
Applications in Pharmaceuticals and Chemical Research
Conformers and tautomers both influence drug design by affecting molecular stability and bioactivity, with conformers representing spatial arrangements around single bonds and tautomers involving proton shifts between isomers. Conformational analysis helps optimize ligand-receptor interactions and pharmacokinetics, while tautomeric equilibrium impacts drug solubility, binding affinity, and metabolic pathways. Techniques like NMR spectroscopy and computational modeling are crucial in identifying preferred conformations and tautomeric forms to enhance pharmaceutical efficacy and chemical synthesis.
Conformer Infographic
 libterm.com
libterm.com