Stereoisomers are molecules that share the same molecular formula and sequence of bonded atoms but differ in the three-dimensional orientations of their atoms in space. These variations can drastically affect the physical properties, biological activity, and chemical reactivity of the compounds. Discover how understanding stereoisomers can enhance your knowledge of chemistry and influence practical applications by reading the rest of the article.
Table of Comparison
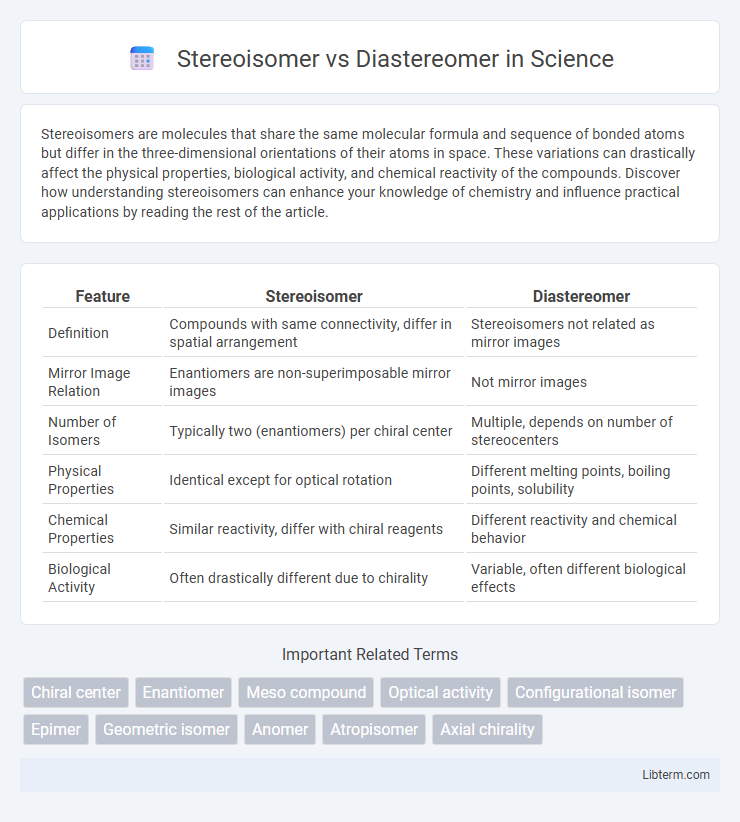
| Feature | Stereoisomer | Diastereomer |
|---|---|---|
| Definition | Compounds with same connectivity, differ in spatial arrangement | Stereoisomers not related as mirror images |
| Mirror Image Relation | Enantiomers are non-superimposable mirror images | Not mirror images |
| Number of Isomers | Typically two (enantiomers) per chiral center | Multiple, depends on number of stereocenters |
| Physical Properties | Identical except for optical rotation | Different melting points, boiling points, solubility |
| Chemical Properties | Similar reactivity, differ with chiral reagents | Different reactivity and chemical behavior |
| Biological Activity | Often drastically different due to chirality | Variable, often different biological effects |
Introduction to Stereoisomers
Stereoisomers are molecules that share the same molecular formula and sequence of bonded atoms but differ in the three-dimensional orientations of their atoms in space. This category includes enantiomers, which are non-superimposable mirror images, and diastereomers, which are stereoisomers not related as mirror images. The distinction between these types of stereoisomers is fundamental in understanding molecular behavior, especially in fields like pharmacology and organic chemistry.
Types of Stereoisomers
Stereoisomers include enantiomers and diastereomers, both differing in spatial arrangement of atoms while sharing the same molecular formula. Enantiomers are non-superimposable mirror images distinguished by opposite configurations at all chiral centers, exhibiting optical activity. Diastereomers differ at one or more (but not all) stereocenters, resulting in distinct physical and chemical properties without being mirror images.
Defining Diastereomers
Diastereomers are stereoisomers that are not mirror images of each other and differ in the configuration of one or more, but not all, stereocenters. Unlike enantiomers, diastereomers exhibit different physical and chemical properties such as melting points, boiling points, and reactivity. Understanding diastereomers is crucial in stereochemistry for predicting molecule behavior and designing selective synthesis pathways.
Structural Differences: Stereoisomers vs Diastereomers
Stereoisomers are molecules with the same molecular formula and sequence of bonded atoms but differ in the three-dimensional orientations of their atoms in space. Diastereomers, a subset of stereoisomers, specifically refer to stereoisomers that are not mirror images of each other and have different physical and chemical properties. Structural differences between stereoisomers and diastereomers arise from the arrangement of atoms around one or more stereocenters without being enantiomeric pairs.
Physical and Chemical Properties Comparison
Stereoisomers and diastereomers differ significantly in their physical properties; stereoisomers, including enantiomers, typically have identical melting points, boiling points, and solubilities, while diastereomers often exhibit distinct melting points and solubilities due to differences in spatial arrangement. Chemically, stereoisomers may react similarly in achiral environments but show different biological activities and optical rotations, whereas diastereomers can have markedly different reactivities and interactions because their spatial configurations influence reaction mechanisms. These variations in physical and chemical behaviors are critical in pharmaceuticals, where stereoisomer purity affects drug efficacy and safety.
Chirality and Optical Activity
Stereoisomers include enantiomers and diastereomers, with chirality as a key factor distinguishing them; enantiomers are non-superimposable mirror images exhibiting opposite optical activity, while diastereomers are stereoisomers that are not mirror images and often show different physical and chemical properties, including variations in optical rotation. Chirality arises from the presence of chiral centers, typically carbon atoms bonded to four distinct substituents, making molecular geometry crucial for stereoisomer classification. Optical activity is measured by the rotation of plane-polarized light, where enantiomers rotate light in equal magnitude but opposite directions, and diastereomers can show weak, strong, or no optical activity depending on their specific stereochemistry.
Methods of Identification and Separation
Stereoisomers, including enantiomers and diastereomers, can be distinguished using polarimetry, where enantiomers rotate plane-polarized light in opposite directions, while diastereomers show different physical properties such as melting points or solubilities. Chromatographic techniques like chiral high-performance liquid chromatography (HPLC) effectively separate enantiomers by exploiting their interactions with chiral stationary phases, whereas diastereomers can often be separated by standard chromatographic methods due to their differing chemical and physical characteristics. Nuclear Magnetic Resonance (NMR) spectroscopy also aids identification by revealing distinct chemical shifts or coupling constants unique to each stereoisomer.
Biological Significance of Diastereomers
Diastereomers exhibit different physical and chemical properties, which significantly influence biological processes such as enzyme specificity and drug efficacy. Unlike stereoisomers including enantiomers, diastereomers can interact distinctively with biological receptors, altering metabolic pathways and pharmacodynamics. These unique interactions contribute to the selective biological activity and toxicity profiles seen in various diastereomeric compounds.
Common Examples in Organic Chemistry
Stereoisomers in organic chemistry include enantiomers like (R)- and (S)-2-butanol, which are non-superimposable mirror images. Diastereomers, such as cis- and trans-2-butene or the sugar isomers D-glucose and D-galactose, differ in configuration at one or more (but not all) stereocenters. These examples highlight the importance of spatial arrangement in molecules affecting properties like optical activity and chemical reactivity.
Summary and Key Takeaways
Stereoisomers are molecules with the same molecular formula and connectivity but differ in the spatial arrangement of atoms, including enantiomers and diastereomers. Diastereomers are a subset of stereoisomers that are not mirror images, differing in configuration at one or more stereocenters. Key distinctions include diastereomers having different physical and chemical properties, unlike enantiomers which share most properties except optical activity.
Stereoisomer Infographic
 libterm.com
libterm.com