Fermentation is a natural metabolic process converting sugars into alcohol, gases, or acids using yeast or bacteria, widely used in food preservation and production. This biochemical reaction enhances flavors, extends shelf life, and boosts the nutritional value of various foods and beverages. Explore the rest of the article to discover how fermentation can transform your culinary experience and health.
Table of Comparison
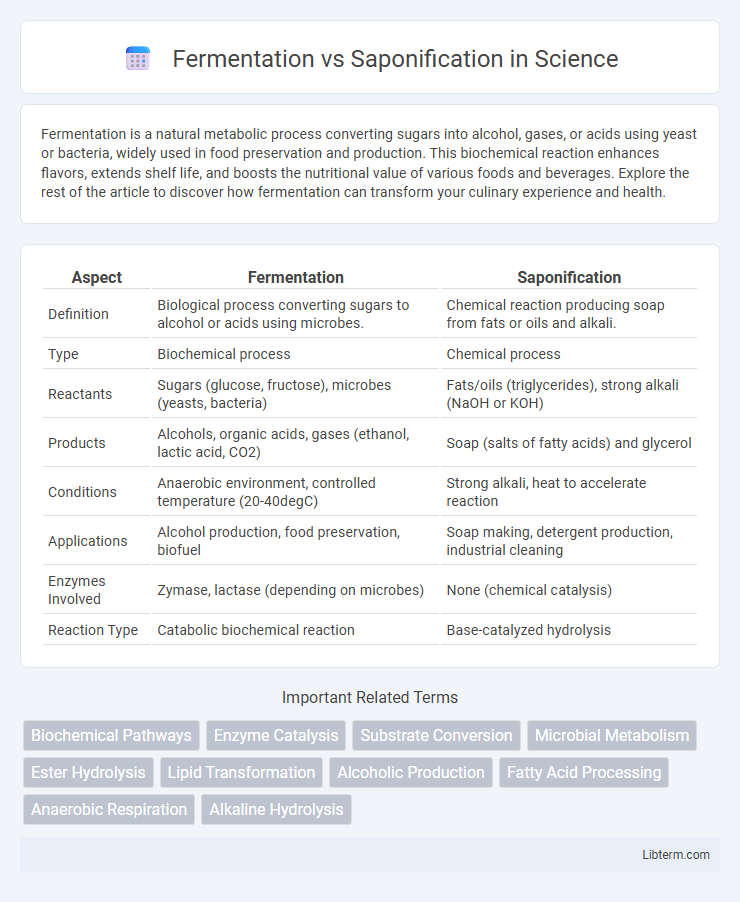
| Aspect | Fermentation | Saponification |
|---|---|---|
| Definition | Biological process converting sugars to alcohol or acids using microbes. | Chemical reaction producing soap from fats or oils and alkali. |
| Type | Biochemical process | Chemical process |
| Reactants | Sugars (glucose, fructose), microbes (yeasts, bacteria) | Fats/oils (triglycerides), strong alkali (NaOH or KOH) |
| Products | Alcohols, organic acids, gases (ethanol, lactic acid, CO2) | Soap (salts of fatty acids) and glycerol |
| Conditions | Anaerobic environment, controlled temperature (20-40degC) | Strong alkali, heat to accelerate reaction |
| Applications | Alcohol production, food preservation, biofuel | Soap making, detergent production, industrial cleaning |
| Enzymes Involved | Zymase, lactase (depending on microbes) | None (chemical catalysis) |
| Reaction Type | Catabolic biochemical reaction | Base-catalyzed hydrolysis |
Introduction to Fermentation and Saponification
Fermentation is a metabolic process where microorganisms like yeast and bacteria convert organic compounds, primarily sugars, into alcohol, gases, or acids under anaerobic conditions. Saponification involves the chemical reaction between a fat or oil and a strong base, such as sodium hydroxide, resulting in the formation of glycerol and soap. These processes are fundamental in industries ranging from biofuel production and food manufacturing to the creation of cleaning agents.
Defining Fermentation: Key Concepts
Fermentation is a metabolic process that converts sugars into gases, acids, or alcohols through the action of microorganisms such as yeast and bacteria. This anaerobic reaction plays a crucial role in producing beverages like beer and wine, as well as foods like yogurt and sauerkraut. Key concepts include substrate utilization, enzymatic activity, and the generation of energy in the absence of oxygen.
Understanding Saponification: Core Principles
Saponification is a chemical reaction where triglycerides from fats or oils react with a strong base, typically sodium hydroxide or potassium hydroxide, to form glycerol and soap. This exothermic process involves the hydrolysis of ester bonds in the triglycerides, resulting in fatty acid salts that possess detergent properties. Understanding the core principles of saponification is crucial for industries like cosmetics and detergents, where precise control over the reactants influences product quality and efficacy.
Historical Context of Fermentation and Saponification
Fermentation, dating back over 9,000 years, played a crucial role in ancient civilizations for producing alcoholic beverages, leavened bread, and preserving food through microbial action. Saponification, developed around 2800 BCE in ancient Babylon, marked a significant advancement in creating soap by reacting fats with alkaline substances, aiding hygiene and cleanliness. Both processes reflect early human innovation in chemistry, shaping cultural practices and daily life through biochemical transformations.
Biological Processes in Fermentation
Fermentation is a biological process where microorganisms such as yeast or bacteria convert sugars into alcohol, acids, or gases under anaerobic conditions, primarily used in food and beverage production. This metabolic process relies on enzymes to break down carbohydrates, generating energy for microbial growth while producing valuable by-products like ethanol or lactic acid. In contrast, saponification is a chemical reaction involving the hydrolysis of fats or oils with an alkali to form soap and glycerol, with no microbial involvement or biological activity.
Chemical Mechanics of Saponification
Saponification is a chemical reaction where triglycerides (fats or oils) react with a strong base, typically sodium hydroxide (NaOH), resulting in the formation of glycerol and soap (salts of fatty acids). This process involves hydrolysis of ester bonds in triglycerides, breaking them down into glycerol and fatty acid anions, which then combine with the alkali metal ions to form soap molecules. Unlike fermentation, which relies on enzymatic conversion by microorganisms, saponification is a purely chemical mechanism driven by alkali-catalyzed ester hydrolysis.
Major Applications: Fermentation vs Saponification
Fermentation is primarily used in the production of alcoholic beverages, biofuels, and pharmaceuticals by converting sugars into alcohol or acids through microbial activity. Saponification is essential in the manufacturing of soaps and detergents by hydrolyzing fats or oils with strong alkali to create glycerol and soap. Both processes serve distinct industrial applications, with fermentation focusing on biochemical transformation and saponification on chemical conversion for cleansing products.
Industrial Impact and Economic Significance
Fermentation drives industrial innovation by producing biofuels, enzymes, and pharmaceuticals, significantly reducing reliance on petrochemicals and enhancing sustainability in sectors such as agriculture and food processing. Saponification, essential in manufacturing soaps and detergents, supports a multibillion-dollar global market by transforming fats and oils into valuable consumer products, driving economic growth in chemical and personal care industries. Both processes influence supply chains, employment rates, and environmental regulations, shaping industrial strategies and economic frameworks worldwide.
Environmental Implications and Sustainability
Fermentation utilizes microbial processes to convert organic materials into useful products, often reducing waste and lowering greenhouse gas emissions compared to chemical methods. Saponification, a chemical reaction involving fats and alkalis to produce soap, can generate waste byproducts and requires energy-intensive conditions, potentially increasing environmental impact. Sustainable practices favor fermentation due to its biodegradability, lower energy consumption, and ability to utilize renewable resources, enhancing overall eco-efficiency.
Choosing Between Fermentation and Saponification
Choosing between fermentation and saponification depends on the desired product and application; fermentation is ideal for producing biofuels, alcohol, and organic acids through microbial metabolism, while saponification is essential for manufacturing soaps and detergents by chemically reacting fats or oils with alkali. Fermentation offers eco-friendly, renewable processes with biological catalysts, whereas saponification provides rapid, controlled chemical conversions suitable for industrial-scale soap production. Consider factors such as raw material availability, process complexity, environmental impact, and end-product specifications to determine the optimal method.
Fermentation Infographic
 libterm.com
libterm.com