Anhydrous substances contain no water molecules within their structure, making them essential in various industrial and chemical processes where moisture can interfere with reactions. Maintaining anhydrous conditions ensures the purity and effectiveness of products like solvents, salts, and pharmaceuticals. Discover how understanding anhydrous materials can benefit your work and explore practical applications in the rest of this article.
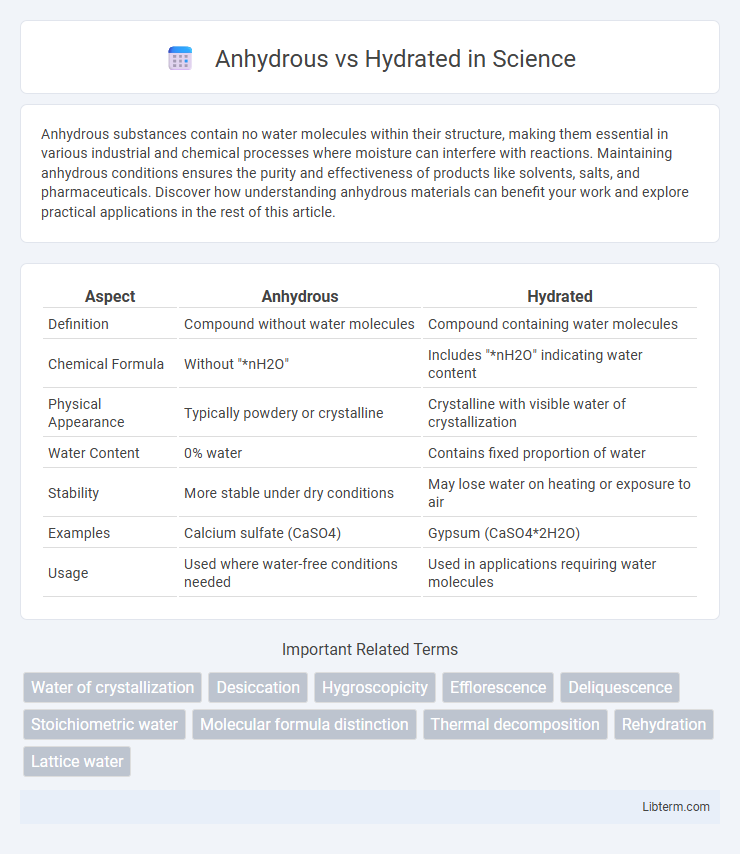
Table of Comparison
| Aspect | Anhydrous | Hydrated |
|---|---|---|
| Definition | Compound without water molecules | Compound containing water molecules |
| Chemical Formula | Without "*nH2O" | Includes "*nH2O" indicating water content |
| Physical Appearance | Typically powdery or crystalline | Crystalline with visible water of crystallization |
| Water Content | 0% water | Contains fixed proportion of water |
| Stability | More stable under dry conditions | May lose water on heating or exposure to air |
| Examples | Calcium sulfate (CaSO4) | Gypsum (CaSO4*2H2O) |
| Usage | Used where water-free conditions needed | Used in applications requiring water molecules |
Understanding Anhydrous and Hydrated Compounds
Anhydrous compounds are substances that do not contain water molecules within their crystalline structure, while hydrated compounds have water molecules chemically bound in their crystal lattice. Understanding the distinction between anhydrous and hydrated forms is crucial in chemical processes, as the presence or absence of water affects the compounds' physical properties, reactivity, and stability. For example, copper sulfate pentahydrate (CuSO4*5H2O) loses its characteristic blue color and becomes anhydrous copper sulfate (CuSO4) when heated, highlighting the importance of water content in compound identity.
Key Differences: Anhydrous vs Hydrated
Anhydrous compounds contain no water molecules within their crystal structure, while hydrated compounds include water molecules chemically bound in their crystalline form. The presence or absence of water significantly affects physical properties such as solubility, stability, and molecular weight, with hydrated forms generally having higher molecular weights due to the incorporated water. Anhydrous chemicals are often preferred in reactions requiring precise stoichiometry or minimal water content to avoid unwanted side reactions.
Chemical Properties of Anhydrous Substances
Anhydrous substances are chemical compounds that lack water molecules in their crystalline structure, resulting in distinct physical and chemical properties compared to their hydrated counterparts. These compounds often exhibit higher reactivity, increased hygroscopicity, and different melting points due to the absence of water. Their ability to act as strong desiccants and participate in unique chemical reactions makes them essential in various industrial and laboratory applications.
Characteristics of Hydrated Compounds
Hydrated compounds contain water molecules chemically bound within their crystalline structure, significantly affecting their physical properties such as color, density, and melting point. These water molecules contribute to the stability and reactivity of the compound, often causing changes in mass during heating or chemical reactions due to dehydration. The presence of water in hydrated compounds can influence solubility and crystallization behavior, distinguishing them from their anhydrous counterparts.
Methods of Preparing Anhydrous and Hydrated Forms
Anhydrous compounds are typically prepared by heating hydrated salts to drive off water molecules through thermal dehydration or by using desiccants such as phosphorus pentoxide to absorb moisture. Hydrated forms are obtained by crystallizing salts from aqueous solutions under controlled humidity, allowing water molecules to integrate into the crystal lattice. Industrial methods often involve precise temperature and environmental controls to switch between anhydrous and hydrated states for specific chemical applications.
Applications in Industry: Anhydrous vs Hydrated
Anhydrous compounds are widely used in industries such as electronics and pharmaceuticals where moisture-sensitive processes are critical, offering enhanced stability and reactivity. Hydrated forms are preferred in agriculture and construction due to their ease of handling, lower flammability, and ability to release water during reactions. Industrial applications leverage the specific properties of anhydrous and hydrated substances to optimize performance in manufacturing, chemical synthesis, and material production.
Environmental Impact: Hydration and Dehydration
Anhydrous compounds contain no water molecules, whereas hydrated compounds include water molecules within their crystal structure, significantly influencing their environmental impact during hydration and dehydration processes. The energy required for dehydration often results in increased carbon emissions due to heating, while hydration can help regulate temperatures and reduce energy consumption by releasing or absorbing heat. Understanding these differences is crucial for industries aiming to minimize ecological footprints through efficient water management in chemical manufacturing and material processing.
Stability and Storage Considerations
Anhydrous compounds exhibit greater chemical stability due to the absence of water molecules, making them less prone to hydrolysis and degradation during storage. Hydrated substances contain bound water, which can promote microbial growth and reduce shelf life, requiring controlled humidity and temperature conditions for preservation. Proper storage of anhydrous materials in airtight, moisture-free environments prevents moisture uptake, whereas hydrated compounds need sealed containers to maintain their hydration state and prevent drying out or caking.
Real-World Examples and Case Studies
Anhydrous compounds, such as anhydrous calcium chloride used in industrial drying processes, demonstrate superior moisture absorption compared to their hydrated counterparts like calcium chloride dihydrate, which is commonly applied in de-icing and dust control. Case studies in pharmaceutical manufacturing highlight the significance of using anhydrous magnesium sulfate to maintain product stability and prevent clumping, whereas hydrated forms risk excess moisture that can degrade active ingredients. Real-world examples in construction show that anhydrous gypsum improves curing times and structural integrity compared to hydrated gypsum, which contains water of crystallization affecting workability and final strength.
Choosing Between Anhydrous and Hydrated Materials
Choosing between anhydrous and hydrated materials depends on the specific requirements of the application, such as moisture sensitivity and stability. Anhydrous substances contain no water and are preferred in processes requiring dry conditions, while hydrated materials include water molecules that can influence solubility and reactivity. Understanding the chemical environment and desired outcome ensures the optimal selection between anhydrous and hydrated compounds.
Anhydrous Infographic
 libterm.com
libterm.com