A prosthetic group is a non-protein molecule tightly bound to a protein that is essential for its biological function, often involved in enzymatic activity or structural stability. These groups can include metal ions, vitamins, or organic compounds, playing a critical role in processes such as electron transfer or substrate binding. Explore the full article to understand how prosthetic groups influence protein function and your health.
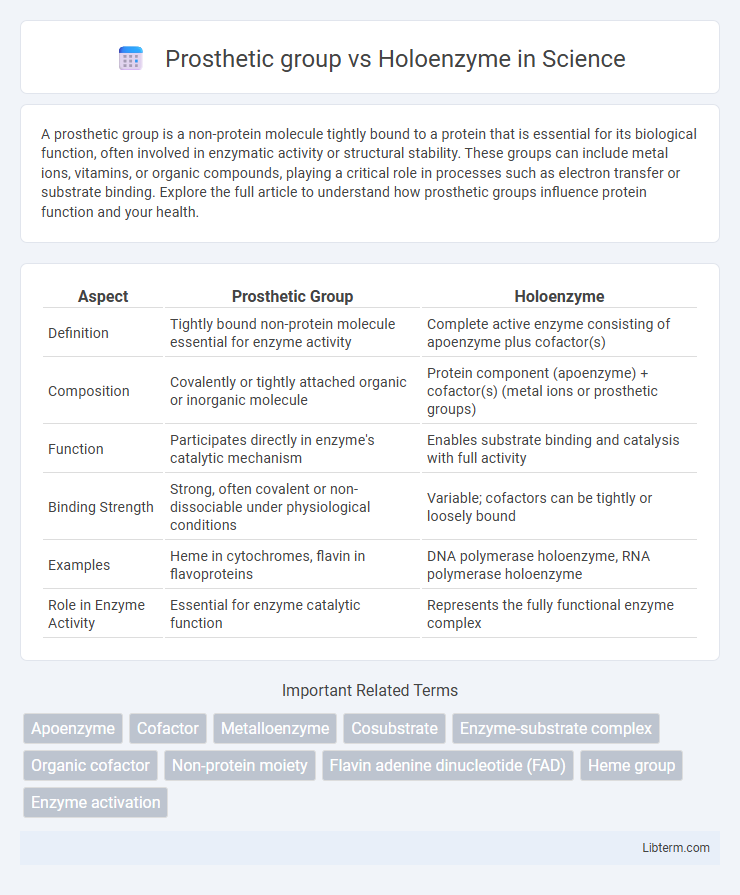
Table of Comparison
| Aspect | Prosthetic Group | Holoenzyme |
|---|---|---|
| Definition | Tightly bound non-protein molecule essential for enzyme activity | Complete active enzyme consisting of apoenzyme plus cofactor(s) |
| Composition | Covalently or tightly attached organic or inorganic molecule | Protein component (apoenzyme) + cofactor(s) (metal ions or prosthetic groups) |
| Function | Participates directly in enzyme's catalytic mechanism | Enables substrate binding and catalysis with full activity |
| Binding Strength | Strong, often covalent or non-dissociable under physiological conditions | Variable; cofactors can be tightly or loosely bound |
| Examples | Heme in cytochromes, flavin in flavoproteins | DNA polymerase holoenzyme, RNA polymerase holoenzyme |
| Role in Enzyme Activity | Essential for enzyme catalytic function | Represents the fully functional enzyme complex |
Introduction to Prosthetic Groups and Holoenzymes
Prosthetic groups are non-protein molecules tightly bound to enzymes, essential for their biological activity and stability. Holoenzymes represent the complete, active enzyme form comprising the apoenzyme and its prosthetic group or cofactor. Understanding the interplay between prosthetic groups and holoenzymes is crucial for studying enzyme functionality and catalytic mechanisms.
Definition of Prosthetic Group
A prosthetic group is a tightly bound, non-polypeptide molecule essential for the biological activity of certain enzymes, often covalently attached to the enzyme's polypeptide chain. It differs from other cofactors by its permanent association with the enzyme, as seen in examples like the heme group in cytochromes. A holoenzyme refers to the complete, active enzyme consisting of the apoenzyme and its prosthetic group or cofactor.
Definition of Holoenzyme
A holoenzyme is a complete, functional enzyme consisting of a core enzyme and its necessary prosthetic group or cofactor, enabling catalytic activity. The prosthetic group is a tightly bound non-protein molecule essential for the enzyme's biological function, often permanently attached to the enzyme. Unlike isolated prosthetic groups, holoenzymes represent the active form of enzymes capable of substrate conversion and biochemical reactions.
Structural Differences Between Prosthetic Groups and Holoenzymes
Prosthetic groups are non-protein molecules tightly bound to enzymes, crucial for their catalytic activity and often permanently attached through covalent or strong non-covalent bonds. Holoenzymes represent the complete, active form of enzymes, encompassing both the apoenzyme (protein portion) and its prosthetic group or cofactor. Structurally, prosthetic groups serve as integral functional components within holoenzymes, whereas holoenzymes portray the entire enzyme complex, combining protein and prosthetic elements for biochemical function.
Functional Role of Prosthetic Groups in Enzymes
Prosthetic groups are non-protein molecules tightly bound to enzymes, essential for their catalytic activity by facilitating electron transfer, stabilizing enzyme structure, or participating directly in the chemical reaction. They enable holoenzymes, which are complete, active enzyme complexes consisting of an apoenzyme (protein part) and its prosthetic group, to perform specific biochemical transformations. The functional role of prosthetic groups is critical in processes like oxidation-reduction reactions, exemplified by heme in cytochromes and flavin adenine dinucleotide (FAD) in oxidoreductases.
Formation and Components of Holoenzymes
Holoenzymes are complete, active enzyme complexes formed by the combination of an apoenzyme (protein part) and its prosthetic group or cofactor, which can be a metal ion or an organic molecule. The prosthetic group is tightly bound to the apoenzyme, often covalently attached, and essential for enzyme activity or substrate binding. Formation of holoenzymes ensures the structural integrity and catalytic functionality necessary for biological processes such as electron transfer or substrate conversion.
Key Examples of Prosthetic Groups
Prosthetic groups are tightly bound non-polypeptide units essential for the biological activity of certain enzymes, with key examples including heme in cytochromes and hemoglobin, flavin adenine dinucleotide (FAD) in oxidases, and biotin in carboxylases. A holoenzyme consists of the apoenzyme (protein part) plus its prosthetic group or cofactor, forming the fully active enzyme complex. Understanding prosthetic groups like metal ions, flavins, or vitamins is crucial for elucidating enzyme mechanisms and functions within holoenzymes.
Biological Significance of Holoenzymes
Holoenzymes consist of an apoenzyme combined with its essential prosthetic group or cofactor, enabling full catalytic activity which is crucial in metabolic pathways and cellular regulation. The biological significance of holoenzymes lies in their ability to perform specific biochemical reactions efficiently, such as DNA replication, transcription, and energy production. Without forming holoenzymes, enzymes remain inactive or have reduced activity, directly impacting physiological processes and organismal homeostasis.
Differences in Cofactor Binding and Enzyme Activity
Prosthetic groups are tightly bound cofactors, often covalently attached to the enzyme, essential for its catalytic activity, whereas holoenzymes refer to the complete enzyme complex, including the apoenzyme and its cofactor, whether tightly or loosely bound. The prosthetic group's permanent attachment enables consistent participation in the enzyme's function, while holoenzyme formation is crucial for activating the apoenzyme and ensuring full enzymatic activity. Differences in cofactor binding affect enzyme stability and substrate interaction, with prosthetic groups providing structural integrity and holoenzymes demonstrating dynamic regulatory potential through cofactor association.
Summary Table: Prosthetic Group vs Holoenzyme
A prosthetic group is a tightly bound non-protein molecule essential for the biological activity of an enzyme, while a holoenzyme refers to the complete, active enzyme comprising the apoenzyme and its prosthetic group or cofactor. Prosthetic groups remain firmly attached to the enzyme during catalysis, whereas holoenzymes represent the functional form necessary for substrate binding and catalytic activity. The distinction lies in the prosthetic group as a component and the holoenzyme as the fully assembled and active catalytic complex.
Prosthetic group Infographic
 libterm.com
libterm.com