Immunoprecipitation is a powerful technique used to isolate and study specific proteins from complex biological samples by employing antibodies that bind uniquely to the target antigen. This method enables detailed analysis of protein-protein interactions, post-translational modifications, and cellular signaling pathways. Explore the article to learn how immunoprecipitation can enhance your research applications and experimental outcomes.
Table of Comparison
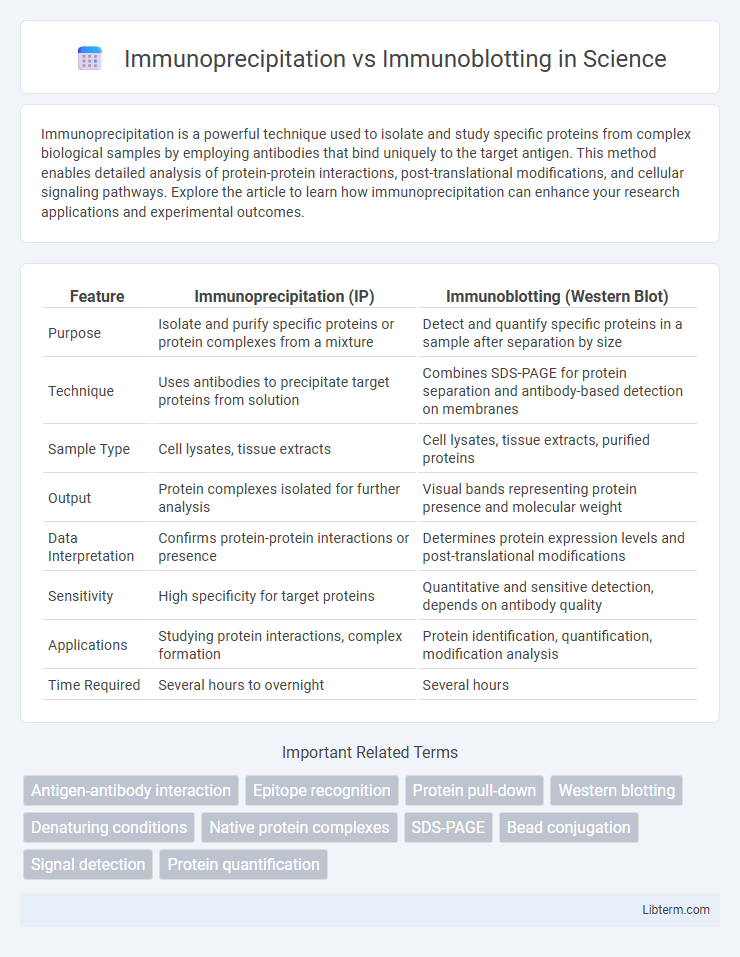
| Feature | Immunoprecipitation (IP) | Immunoblotting (Western Blot) |
|---|---|---|
| Purpose | Isolate and purify specific proteins or protein complexes from a mixture | Detect and quantify specific proteins in a sample after separation by size |
| Technique | Uses antibodies to precipitate target proteins from solution | Combines SDS-PAGE for protein separation and antibody-based detection on membranes |
| Sample Type | Cell lysates, tissue extracts | Cell lysates, tissue extracts, purified proteins |
| Output | Protein complexes isolated for further analysis | Visual bands representing protein presence and molecular weight |
| Data Interpretation | Confirms protein-protein interactions or presence | Determines protein expression levels and post-translational modifications |
| Sensitivity | High specificity for target proteins | Quantitative and sensitive detection, depends on antibody quality |
| Applications | Studying protein interactions, complex formation | Protein identification, quantification, modification analysis |
| Time Required | Several hours to overnight | Several hours |
Introduction to Immunoprecipitation and Immunoblotting
Immunoprecipitation is a targeted technique used to isolate a specific antigen from a complex protein mixture using an antibody that binds to the antigen, forming an immune complex that can be precipitated and analyzed. Immunoblotting, also known as Western blotting, involves the separation of proteins by gel electrophoresis, followed by transfer to a membrane and detection using specific antibodies to identify target proteins. Both methods are essential in molecular biology for studying protein-protein interactions, post-translational modifications, and verifying protein expression.
Principles of Immunoprecipitation
Immunoprecipitation relies on antigen-antibody specificity to isolate a target protein from a complex mixture by forming an immune complex that can be captured using protein A/G beads. This principle enables selective enrichment and analysis of proteins, including their complexes and post-translational modifications. Immunoprecipitation is distinct from immunoblotting, which detects proteins separated by gel electrophoresis using antibody-based detection.
Principles of Immunoblotting
Immunoblotting, or Western blotting, relies on the principle of protein separation by electrophoresis followed by transfer to a membrane for antibody detection. Target proteins are identified through specific antigen-antibody interactions, where primary antibodies bind to the protein of interest and secondary antibodies conjugated to enzymes or fluorophores enable visualization. This technique provides qualitative and semi-quantitative analysis of protein expression, post-translational modifications, and protein-protein interactions.
Key Differences Between Immunoprecipitation and Immunoblotting
Immunoprecipitation isolates a specific antigen from a complex mixture using an antibody to form an antigen-antibody complex that can be pulled down and analyzed, whereas immunoblotting detects proteins separated by gel electrophoresis through antibody binding on a membrane. Immunoprecipitation is primarily used to study protein interactions and modifications, while immunoblotting quantitatively and qualitatively analyzes protein expression levels. The key difference lies in immunoprecipitation capturing proteins from solution, contrasting with immunoblotting's detection of proteins immobilized on a membrane after electrophoretic separation.
Applications in Protein Analysis
Immunoprecipitation isolates specific proteins or protein complexes from cell lysates, enabling the study of protein-protein interactions and post-translational modifications in protein analysis. Immunoblotting, or Western blotting, quantitatively detects and characterizes proteins after separation by gel electrophoresis, facilitating identification and expression level analysis of target proteins. Combining both techniques provides comprehensive insights into protein function, interactions, and modifications in molecular biology research.
Antibody Selection and Specificity
Antibody selection and specificity are critical in both immunoprecipitation (IP) and immunoblotting (Western blot), but each technique demands different considerations. Immunoprecipitation requires antibodies with high affinity and specificity to capture the native form of the target protein from complex mixtures without cross-reactivity, often favoring monoclonal antibodies or affinity-purified polyclonals. Immunoblotting demands antibodies that recognize denatured epitopes on proteins separated by SDS-PAGE, where antibody specificity is essential to distinguish target proteins from background signals, commonly utilizing validated monoclonal antibodies or well-characterized polyclonal antibodies.
Sample Preparation Techniques
Immunoprecipitation (IP) requires lysing cells with non-denaturing buffers to preserve protein-protein interactions, often involving gentle detergents like NP-40 or Triton X-100. Immunoblotting, or Western blotting, demands protein denaturation using SDS-containing buffers to linearize proteins before gel electrophoresis. Optimal sample preparation in IP maintains native protein complexes, whereas immunoblotting focuses on individual protein detection through denaturation and transfer onto membranes.
Advantages and Limitations of Each Method
Immunoprecipitation enables the isolation of specific proteins or protein complexes from a mixture, providing insights into protein interactions and post-translational modifications, but it can be limited by antibody specificity and the requirement for relatively large sample quantities. Immunoblotting, commonly known as Western blotting, offers precise detection and quantification of target proteins with high sensitivity and the ability to analyze protein size, yet it lacks the capability to assess protein interactions directly and is dependent on effective protein transfer and antibody binding. Combining both methods enhances protein analysis by leveraging immunoprecipitation's enrichment strengths and immunoblotting's detection accuracy, though challenges remain regarding antibody quality and procedural complexity.
Troubleshooting Common Issues
Immunoprecipitation troubleshooting often involves optimizing antibody specificity and protein complex stability to reduce non-specific binding and improve yield, while ensuring adequate washing steps to minimize background noise. Immunoblotting challenges frequently include weak signal detection, which can be addressed by verifying antibody quality, optimizing blocking conditions, and adjusting transfer parameters for efficient protein transfer onto membranes. Both techniques benefit from meticulous sample preparation and using proper controls to identify and resolve artifacts or inconsistent results.
Future Perspectives in Protein Detection Methods
Emerging protein detection methods are integrating immunoprecipitation with advanced mass spectrometry and high-throughput immunoblotting to enhance sensitivity and specificity. Future perspectives emphasize automation, multiplexing capabilities, and real-time quantification to accelerate biomarker discovery and personalized medicine. Novel biosensor technologies combined with AI-driven data analysis promise transformative improvements in protein-protein interaction studies and disease diagnostics.
Immunoprecipitation Infographic
 libterm.com
libterm.com