Antibodies are specialized proteins produced by the immune system to identify and neutralize harmful pathogens such as bacteria and viruses. Their unique structure allows them to bind specifically to antigens, triggering an immune response that protects your body from infections. Discover how antibodies work and their crucial role in health by reading the rest of this article.
Table of Comparison
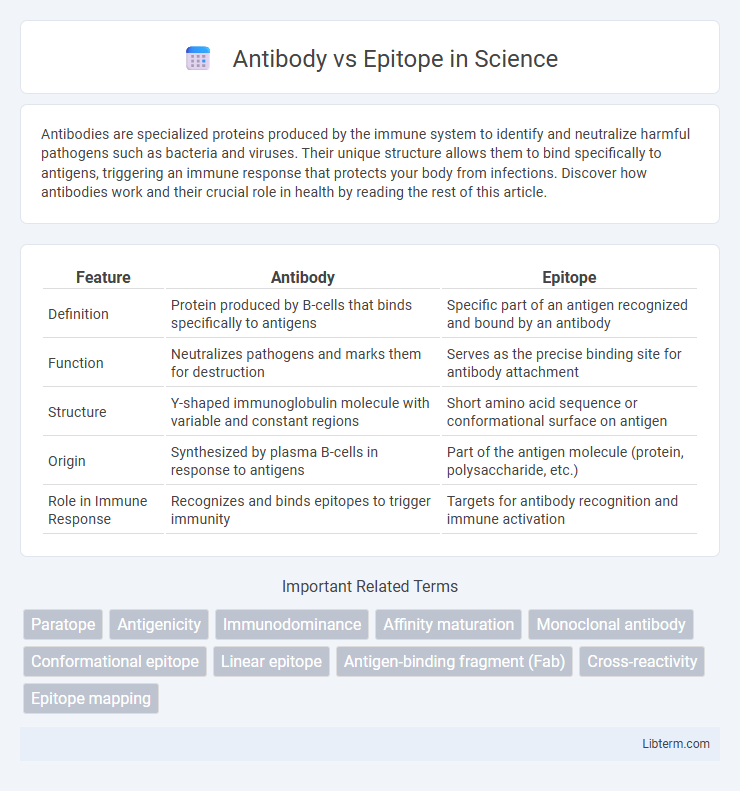
| Feature | Antibody | Epitope |
|---|---|---|
| Definition | Protein produced by B-cells that binds specifically to antigens | Specific part of an antigen recognized and bound by an antibody |
| Function | Neutralizes pathogens and marks them for destruction | Serves as the precise binding site for antibody attachment |
| Structure | Y-shaped immunoglobulin molecule with variable and constant regions | Short amino acid sequence or conformational surface on antigen |
| Origin | Synthesized by plasma B-cells in response to antigens | Part of the antigen molecule (protein, polysaccharide, etc.) |
| Role in Immune Response | Recognizes and binds epitopes to trigger immunity | Targets for antibody recognition and immune activation |
Introduction to Antibodies and Epitopes
Antibodies are specialized proteins produced by the immune system to recognize and bind specific foreign molecules, known as antigens. Epitopes are the distinct molecular regions on an antigen that antibodies specifically target and attach to during an immune response. Understanding the precise interaction between antibodies and epitopes is essential for advancements in vaccine development and diagnostic testing.
Defining Antibodies: Structure and Function
Antibodies are Y-shaped glycoproteins composed of two heavy chains and two light chains, forming variable regions that specifically recognize antigens. Their primary function is to neutralize pathogens by binding to epitopes--distinct molecular structures on antigens--facilitating immune responses such as opsonization and complement activation. The structural specificity of antibodies enables precise targeting of epitopes, crucial for adaptive immunity and vaccine development.
Understanding Epitopes: The Antigenic Determinant
Epitopes, also known as antigenic determinants, are specific regions on an antigen recognized and bound by antibodies during the immune response. These molecular structures, typically composed of amino acid sequences or carbohydrate moieties, determine the specificity of antibody-antigen interactions, influencing immune recognition and vaccine design. Understanding epitopes is crucial for developing targeted immunotherapies, as they dictate the precision with which antibodies can neutralize pathogens or mark them for destruction.
Types of Epitopes: Linear vs Conformational
Epitopes, the specific parts of an antigen recognized by antibodies, are categorized into linear and conformational types. Linear epitopes consist of continuous amino acid sequences and can be identified through peptide synthesis, whereas conformational epitopes are formed by amino acids brought together in the three-dimensional structure, requiring the native protein conformation. Understanding the distinction between these epitope types is crucial for antibody design, vaccine development, and diagnostic applications.
Antibody-Epitope Binding Mechanism
The antibody-epitope binding mechanism relies on highly specific interactions between the antigen-binding site of the antibody and the epitope, which is a distinct molecular structure on the antigen. This binding occurs through non-covalent forces such as hydrogen bonds, Van der Waals forces, electrostatic interactions, and hydrophobic effects, allowing precise recognition and strong affinity. Structural complementarity and conformational flexibility enable antibodies to selectively bind linear or conformational epitopes, facilitating immune response and pathogen neutralization.
Specificity and Diversity of Antibody-Epitope Interaction
Antibodies exhibit high specificity by recognizing distinct epitopes, which are specific molecular regions on antigens. The diversity of antibody-epitope interactions results from variable regions in antibodies, enabling the immune system to target a broad array of epitopes with precise binding affinity. This specificity and diversity are crucial for effective immune surveillance and pathogen neutralization.
Role of Antibodies and Epitopes in Immune Response
Antibodies, also known as immunoglobulins, are proteins produced by B cells that specifically recognize and bind to epitopes, the distinct molecular structures on antigens. This binding facilitates the neutralization of pathogens and marks them for destruction by other immune cells, playing a crucial role in adaptive immunity. Epitopes, whether linear or conformational, determine the specificity of the immune response by guiding antibody targeting and immune memory formation.
Applications in Diagnostics and Therapeutics
Antibodies are proteins that specifically bind to epitopes, the distinct molecular regions on antigens, enabling highly selective targeting in diagnostic assays such as ELISA and immunohistochemistry. In therapeutics, monoclonal antibodies designed to recognize specific epitopes facilitate targeted drug delivery, immune modulation, and cancer treatment through mechanisms like antibody-dependent cellular cytotoxicity. Epitope mapping enhances the precision of antibody development, improving the sensitivity and specificity of both diagnostic tools and therapeutic agents.
Challenges in Mapping Epitopes and Designing Antibodies
Mapping epitopes involves identifying precise antigenic sites recognized by antibodies, which is complicated by the conformational flexibility and heterogeneous nature of epitopes. Designing antibodies requires overcoming challenges such as ensuring high specificity and affinity while minimizing cross-reactivity and immunogenicity. Advanced computational modeling and high-throughput screening are crucial tools to address these complexities in antibody and epitope interaction studies.
Future Directions in Antibody-Epitope Research
Future directions in antibody-epitope research emphasize the integration of high-throughput sequencing and artificial intelligence to map epitope landscapes with unprecedented precision. Advancements in structural biology and machine learning models aim to predict antibody-epitope interactions, enhancing vaccine design and targeted immunotherapies. Emerging techniques such as single-cell multi-omics and CRISPR-based screening are poised to revolutionize personalized medicine through tailored antibody development.
Antibody Infographic
 libterm.com
libterm.com