Epi is a powerful tool designed to streamline data collection and analysis in epidemiological research, enhancing accuracy and efficiency. Its user-friendly interface allows health professionals to manage study data seamlessly, ensuring that critical insights are easily accessible. Explore the rest of the article to discover how Epi can optimize your research processes and improve public health outcomes.
Table of Comparison
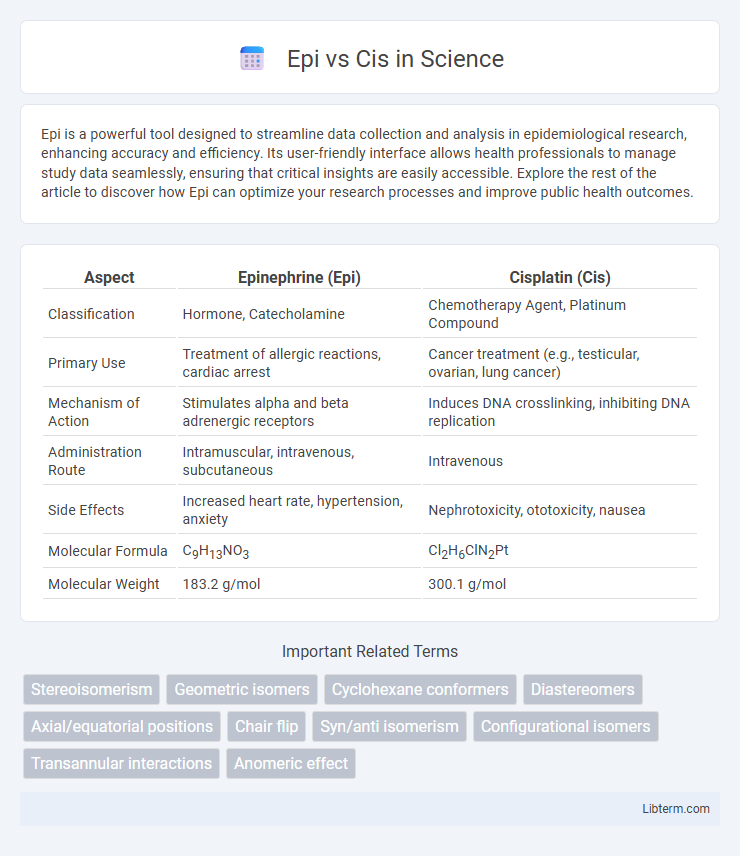
| Aspect | Epinephrine (Epi) | Cisplatin (Cis) |
|---|---|---|
| Classification | Hormone, Catecholamine | Chemotherapy Agent, Platinum Compound |
| Primary Use | Treatment of allergic reactions, cardiac arrest | Cancer treatment (e.g., testicular, ovarian, lung cancer) |
| Mechanism of Action | Stimulates alpha and beta adrenergic receptors | Induces DNA crosslinking, inhibiting DNA replication |
| Administration Route | Intramuscular, intravenous, subcutaneous | Intravenous |
| Side Effects | Increased heart rate, hypertension, anxiety | Nephrotoxicity, ototoxicity, nausea |
| Molecular Formula | C9H13NO3 | Cl2H6ClN2Pt |
| Molecular Weight | 183.2 g/mol | 300.1 g/mol |
Understanding Epi and Cis: Basic Definitions
Epi and Cis refer to specific types of stereochemical relationships in molecules, where "cis" indicates substituents on the same side of a reference plane, and "epi" denotes a related concept often found in epimers--diastereomers differing at a single stereogenic center. Understanding these terms is crucial in organic chemistry, as cis isomers typically exhibit different physical and chemical properties compared to trans isomers, while epimers influence sugar and steroid configurations. Mastery of epi and cis definitions aids in predicting molecular behavior, reactivity, and biological activity, essential for applications in drug design and synthesis.
Structural Differences between Epi and Cis
Epi and Cis isomers differ primarily in the spatial arrangement of substituent groups around a ring or double bond, impacting their chemical properties and reactivity. In Epi isomers, substituents are positioned on opposite sides, leading to greater steric separation, whereas Cis isomers have substituents on the same side, causing increased steric hindrance. These structural differences influence their molecular geometry, affecting polarity, boiling points, and interaction with biological targets.
Stereochemistry: Epi vs Cis
Epi and Cis stereochemistry describe different spatial arrangements of substituents around a chiral center or double bond. In Epi isomers, substituents are positioned on opposite sides, often leading to distinct stereochemical configurations compared to Cis isomers, where substituents lie on the same side. This difference significantly influences molecular properties such as reactivity, polarity, and biological activity.
Chemical Properties Comparison
Epinephrine (Epi) and Cisatracurium (Cis) differ significantly in chemical properties, with epinephrine being a catecholamine composed of a benzene ring with two hydroxyl groups and an amine side chain, contributing to its high polarity and water solubility. Cisatracurium is a bisquaternary ammonium compound with complex ester and amide linkages, giving it a larger molecular weight and lower polarity compared to epinephrine. These structural differences influence their pharmacokinetics and receptor binding characteristics, with epinephrine acting primarily on adrenergic receptors and cisatracurium targeting nicotinic acetylcholine receptors.
Biological Significance of Epi and Cis Isomers
Epi and cis isomers differ in the spatial arrangement of atoms, profoundly impacting their biological functions and molecular recognition. Epi isomers often exhibit distinct bioactivity and enzyme specificity due to altered stereochemistry influencing binding affinity. Cis isomers typically show unique membrane permeability and receptor interaction profiles, making their biological significance critical in drug design and metabolic pathways.
Occurrence in Nature: Epi vs Cis Compounds
Epi and cis compounds differ primarily in their spatial arrangement of atoms, influencing their natural occurrence and biological activity. Cis compounds often appear in natural products such as unsaturated fatty acids where the same side positioning around a double bond affects molecular shape and function. Epi compounds, a subset of stereoisomers differing in the configuration of one stereogenic center, are less common but important in natural products like certain alkaloids and steroids, contributing to diverse biochemical properties.
Methods for Identifying Epi and Cis Isomers
Methods for identifying epi and cis isomers primarily involve spectroscopic techniques such as nuclear magnetic resonance (NMR) spectroscopy, which distinguishes isomers based on differences in chemical shifts and coupling constants. Chromatographic methods like gas chromatography (GC) and high-performance liquid chromatography (HPLC) enable separation and quantification of epi and cis isomers due to their distinct polarity and retention times. Advanced techniques such as X-ray crystallography provide definitive structural confirmation by directly observing the spatial arrangement of atoms in epi and cis configurations.
Applications in Pharmaceuticals and Industry
Epichlorohydrin (Epi) is widely used in the pharmaceutical industry as a key intermediate for synthesizing epoxy resins and glycidyl ethers, essential for drug delivery systems and medical device coatings. Cis-isomers, particularly in cis-platin and related compounds, serve as crucial chemotherapeutic agents due to their ability to bind DNA and inhibit cancer cell growth. Industrial applications leverage the reactive epoxide groups in Epi for producing adhesives, sealants, and water treatment chemicals, while cis-configured compounds are vital for creating stereospecific drugs and polymers with precise molecular arrangements.
Synthesis Techniques: Epi vs Cis
Epitaxial (Epi) synthesis techniques involve the growth of crystalline layers on a substrate, enabling precise control of layer thickness and composition for high-quality semiconductor devices. Chemical vapor deposition (CVD) and molecular beam epitaxy (MBE) are common Epi methods that promote uniform, defect-free films with tailored electronic properties. In contrast, conventional Czochralski (Cis) synthesis primarily focuses on bulk crystal pulling, emphasizing large single-crystal growth but with less fine control over layer composition or nanoscale features.
Challenges and Advances in Epi vs Cis Research
Epi versus Cis research faces significant challenges including the complexity of epigenetic modifications and the difficulty in distinguishing causal relationships from mere associations within cis-regulatory elements. Advances in high-throughput sequencing technologies and CRISPR-based epigenome editing have enabled more precise mapping and functional analysis of epi- and cis-regulatory mechanisms. Integrating multi-omics data sets accelerates the discovery of dynamic regulatory networks underlying gene expression variations in health and disease contexts.
Epi Infographic
 libterm.com
libterm.com