Amphiphilic molecules possess both hydrophilic (water-attracting) and hydrophobic (water-repelling) properties, allowing them to interact with diverse environments and form structures like micelles and bilayers. These unique characteristics make amphiphilic compounds essential in applications ranging from drug delivery to detergents and membrane biology. Explore the rest of the article to discover how amphiphilic molecules impact various scientific fields and your everyday life.
Table of Comparison
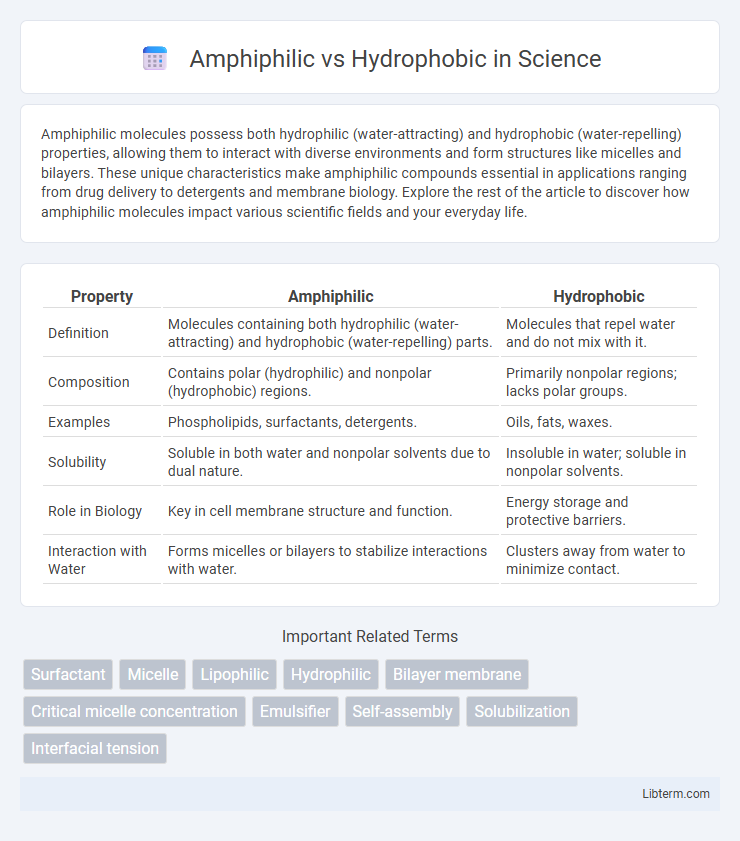
| Property | Amphiphilic | Hydrophobic |
|---|---|---|
| Definition | Molecules containing both hydrophilic (water-attracting) and hydrophobic (water-repelling) parts. | Molecules that repel water and do not mix with it. |
| Composition | Contains polar (hydrophilic) and nonpolar (hydrophobic) regions. | Primarily nonpolar regions; lacks polar groups. |
| Examples | Phospholipids, surfactants, detergents. | Oils, fats, waxes. |
| Solubility | Soluble in both water and nonpolar solvents due to dual nature. | Insoluble in water; soluble in nonpolar solvents. |
| Role in Biology | Key in cell membrane structure and function. | Energy storage and protective barriers. |
| Interaction with Water | Forms micelles or bilayers to stabilize interactions with water. | Clusters away from water to minimize contact. |
Understanding Amphiphilic and Hydrophobic Molecules
Amphiphilic molecules possess both hydrophilic (water-attracting) and hydrophobic (water-repelling) properties, allowing them to interact with diverse environments, such as forming micelles and bilayers in aqueous solutions. Hydrophobic molecules repel water and tend to aggregate to minimize contact with water, influencing processes like membrane formation and protein folding. Understanding the distinct behaviors of amphiphilic and hydrophobic molecules is crucial for applications in drug delivery, detergency, and nanotechnology.
Key Differences Between Amphiphilic and Hydrophobic Properties
Amphiphilic molecules possess both hydrophilic (water-attracting) and hydrophobic (water-repelling) regions, enabling them to interact with polar and nonpolar substances, which is crucial in forming micelles and bilayers in biological membranes. Hydrophobic molecules, in contrast, lack affinity for water and tend to aggregate to minimize contact with aqueous environments, driving phenomena like protein folding and lipid membrane integrity. The key difference lies in amphiphilic compounds' dual affinity that supports emulsification and self-assembly, whereas hydrophobic compounds chiefly drive phase separation due to their water-repelling nature.
Chemical Structures: Amphiphilic vs Hydrophobic Compounds
Amphiphilic compounds possess distinct hydrophilic (polar) and hydrophobic (nonpolar) regions within their molecular structures, enabling them to interact with both aqueous and lipid environments. Hydrophobic compounds consist primarily of nonpolar hydrocarbon chains or rings, which repel water and do not dissolve in polar solvents. The dual nature of amphiphilic molecules, such as phospholipids with polar heads and nonpolar tails, contrasts sharply with the uniformly nonpolar character of hydrophobic molecules, influencing their behavior in biological membranes and surfactant applications.
Interactions with Water: Solubility and Behavior
Amphiphilic molecules contain both hydrophobic and hydrophilic regions, enabling them to interact with water by forming micelles or bilayers, which enhances solubility and stabilizes emulsions. Hydrophobic molecules repel water and do not dissolve, instead aggregating to minimize contact with water, influencing phenomena such as phase separation and membrane formation. The balance of hydrophilic and hydrophobic interactions determines molecular behavior in aqueous environments, crucial for applications in drug delivery and detergency.
Biological Roles of Amphiphilic and Hydrophobic Molecules
Amphiphilic molecules, possessing both hydrophilic and hydrophobic regions, play crucial roles in forming biological membranes, enabling selective permeability and cellular compartmentalization. Hydrophobic molecules primarily function in energy storage and insulation, forming lipid droplets that serve as reservoirs of metabolic fuel. The interplay between amphiphilic and hydrophobic properties is essential for processes like membrane protein function and cellular signaling.
Applications in Drug Delivery and Nanotechnology
Amphiphilic molecules possess both hydrophilic and hydrophobic domains, enabling them to self-assemble into micelles and liposomes that enhance drug solubility and targeted delivery in nanotechnology. Hydrophobic compounds, when encapsulated within amphiphilic carriers, improve bioavailability and reduce toxicity by protecting drugs from premature degradation. This dual characteristic is crucial for designing advanced nanocarriers in controlled release systems and precision medicine applications.
Environmental Impact and Functions
Amphiphilic molecules possess both hydrophobic and hydrophilic properties, enabling them to interact with diverse environmental media and facilitate pollutant breakdown through emulsification and solubilization. Hydrophobic substances repel water, often leading to accumulation in ecosystems and long-lasting contamination due to poor biodegradability and bioaccumulation in aquatic organisms. Understanding these differences is crucial for designing effective bioremediation strategies and assessing ecological risks associated with chemical pollutants.
Examples of Amphiphilic and Hydrophobic Substances
Amphiphilic substances include phospholipids, which form cell membranes by containing both hydrophobic tails and hydrophilic heads, and detergents like sodium dodecyl sulfate that emulsify oils in water. Hydrophobic substances encompass oils such as vegetable oil and hydrocarbons like hexane, which repel water and do not dissolve in aqueous solutions. Surfactants exhibit amphiphilic properties allowing them to interact with both hydrophobic and hydrophilic molecules efficiently.
Experimental Methods to Distinguish Amphiphilic and Hydrophobic Molecules
Experimental methods to distinguish amphiphilic and hydrophobic molecules often involve surface tension measurements and contact angle goniometry, where amphiphilic molecules typically reduce surface tension more significantly due to their dual affinity for water and oils. Spectroscopic techniques like NMR and fluorescence spectroscopy can identify molecular interactions with solvents, showing amphiphilic molecules' ability to associate with both polar and nonpolar environments. Additionally, partition coefficient analysis between aqueous and organic phases quantitatively differentiates amphiphilic molecules, which distribute across both phases, from hydrophobic molecules that primarily remain in nonpolar solvents.
Future Trends and Research Directions
Emerging research in amphiphilic materials focuses on their tunable self-assembly for targeted drug delivery and advanced nanotechnology applications, driven by innovations in molecular design and responsive behavior. Hydrophobic substances continue to be explored for sustainable water-repellent coatings and environmentally friendly separations, leveraging new surface modification techniques and bioinspired models. Future trends predict hybrid amphiphilic-hydrophobic systems enabling multifunctional performance in smart materials, energy storage, and biomedical engineering.
Amphiphilic Infographic
 libterm.com
libterm.com