Visible light is the portion of the electromagnetic spectrum that the human eye can detect, ranging from approximately 400 to 700 nanometers in wavelength. It enables us to perceive colors and shapes, playing a crucial role in daily activities such as reading, driving, and recognizing faces. Discover how visible light impacts your environment and technology by exploring the rest of this article.
Table of Comparison
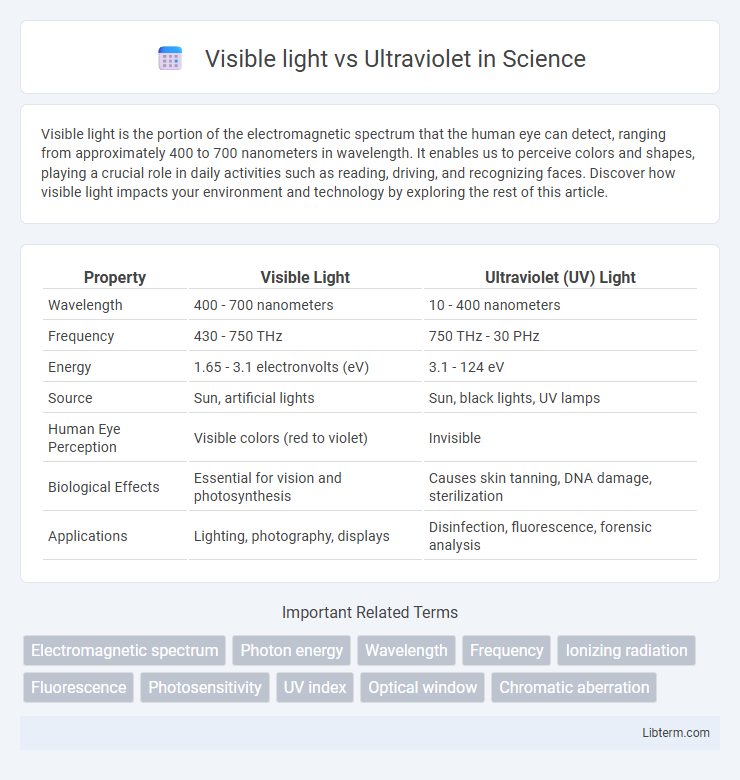
| Property | Visible Light | Ultraviolet (UV) Light |
|---|---|---|
| Wavelength | 400 - 700 nanometers | 10 - 400 nanometers |
| Frequency | 430 - 750 THz | 750 THz - 30 PHz |
| Energy | 1.65 - 3.1 electronvolts (eV) | 3.1 - 124 eV |
| Source | Sun, artificial lights | Sun, black lights, UV lamps |
| Human Eye Perception | Visible colors (red to violet) | Invisible |
| Biological Effects | Essential for vision and photosynthesis | Causes skin tanning, DNA damage, sterilization |
| Applications | Lighting, photography, displays | Disinfection, fluorescence, forensic analysis |
Introduction to Visible Light and Ultraviolet
Visible light is the portion of the electromagnetic spectrum that is detectable by the human eye, with wavelengths ranging approximately from 380 to 750 nanometers. Ultraviolet (UV) light has shorter wavelengths, from about 10 to 400 nanometers, and lies just beyond the visible spectrum, meaning it is invisible to humans. Both types of light carry energy, with ultraviolet light having higher energy and greater potential to cause chemical changes in materials.
Wavelength Differences: Visible Light vs Ultraviolet
Visible light spans wavelengths from approximately 400 to 700 nanometers, whereas ultraviolet (UV) light ranges from about 10 to 400 nanometers. The shorter wavelength of UV light results in higher energy photons capable of causing chemical reactions and biological effects like skin damage. In contrast, visible light's longer wavelengths are responsible for human color perception and are less energetic, making them safer for daily exposure.
Energy Levels: Comparing Visible Light and UV Rays
Visible light comprises wavelengths approximately between 400 and 700 nanometers, carrying energy levels that range from about 1.7 to 3.1 electron volts (eV), making it essential for human vision. Ultraviolet (UV) rays have shorter wavelengths, roughly 10 to 400 nanometers, which correlate with higher energy levels from 3.1 eV up to 124 eV, enabling them to cause chemical reactions such as skin tanning or damage. The higher photon energy in UV radiation makes it more capable of ionizing atoms and molecules, explaining its greater potential for biological effects compared to visible light.
Sources of Visible and Ultraviolet Light
Visible light primarily originates from natural sources like the sun and artificial sources such as incandescent bulbs, LEDs, and fluorescent lamps, which emit wavelengths detectable by the human eye. Ultraviolet (UV) light is generated mainly by the sun as well, with artificial sources including mercury-vapor lamps, black lights, and specialized UV LEDs used for sterilization and curing processes. Both types of light have distinct wavelength ranges; visible light spans approximately 400-700 nanometers, while ultraviolet light covers 10-400 nanometers, influencing their sources and applications.
Interaction with Matter: Reflection, Absorption, and Transmission
Visible light interacts with matter primarily through reflection, absorption, and transmission, with materials like glass allowing most visible wavelengths to pass through while reflecting some. Ultraviolet (UV) light tends to be absorbed more strongly by many substances, causing electronic transitions in molecules and breaking chemical bonds, which results in reduced transmission and increased absorption in materials such as plastics and biological tissues. Reflection of UV light varies depending on the surface properties but generally contributes less to material interaction compared to the dominant absorption effects.
Biological Effects: Visible Light vs Ultraviolet Exposure
Ultraviolet (UV) light penetrates the skin more deeply than visible light, causing DNA damage, sunburn, and increasing the risk of skin cancer due to its higher energy wavelengths. Visible light primarily affects the eyes by potentially inducing retinal stress and influencing circadian rhythms, but it does not cause the same extent of cellular damage as UV radiation. Prolonged UV exposure triggers skin aging and immunosuppression, whereas visible light exposure mainly impacts visual perception and hormonal regulation without direct genetic harm.
Practical Applications of Visible and Ultraviolet Light
Visible light enables practical applications such as photography, illumination, and visual displays, capitalizing on its ability to interact with human vision. Ultraviolet (UV) light is extensively used for sterilization, forensic analysis, and fluorescent detection due to its higher energy and shorter wavelength compared to visible light. Innovations in UV curing technologies and visible light communication further highlight the distinct advantages of each spectrum in industry and science.
Safety Precautions: Protection from Ultraviolet Radiation
Ultraviolet (UV) radiation poses significant risks to skin and eye health, requiring stringent safety measures such as wearing broad-spectrum sunscreen with an SPF of 30 or higher, UV-blocking sunglasses, and protective clothing. Unlike visible light, which is largely harmless in typical exposure, UV rays can cause sunburn, premature aging, and increase the risk of skin cancer, making vigilant protection essential. Limiting direct exposure during peak sunlight hours and using physical barriers like shade or UV-blocking windows further enhances safety against harmful UV radiation.
Detection and Measurement Techniques
Visible light detection primarily relies on photodiodes, charge-coupled devices (CCDs), and photomultiplier tubes (PMTs) that convert light photons into electrical signals within the 400-700 nm wavelength range. Ultraviolet (UV) detection utilizes specialized sensors such as UV photodiodes, scintillators, and vacuum phototubes sensitive to shorter wavelengths from 10 to 400 nm, often requiring materials like silicon carbide or diamond for enhanced responsiveness. Measurement techniques for both involve spectroradiometers and filter-based radiometers, with UV instruments frequently incorporating atmospheric correction algorithms to account for absorption and scattering effects.
Conclusion: Key Differences and Impacts
Visible light, with wavelengths ranging from approximately 400 to 700 nanometers, enables human vision and supports photosynthesis, whereas ultraviolet (UV) light, spanning 10 to 400 nanometers, carries higher energy capable of causing molecular damage and skin cancer. The key differences lie in their energy levels, biological effects, and applications, where visible light is essential for everyday life and UV light is harnessed for sterilization and medical treatments but requires caution due to its harmful impacts. Understanding these distinctions guides safe usage and maximizes benefits across health, technology, and environmental sectors.
Visible light Infographic
 libterm.com
libterm.com