Immunotolerant refers to a state where the immune system does not mount a response against specific antigens, allowing the body to coexist peacefully with these substances. This mechanism is crucial in preventing autoimmune diseases and plays a significant role in organ transplantation and chronic infections. Discover how understanding immunotolerance can enhance your approach to immune-related health issues by reading the full article.
Table of Comparison
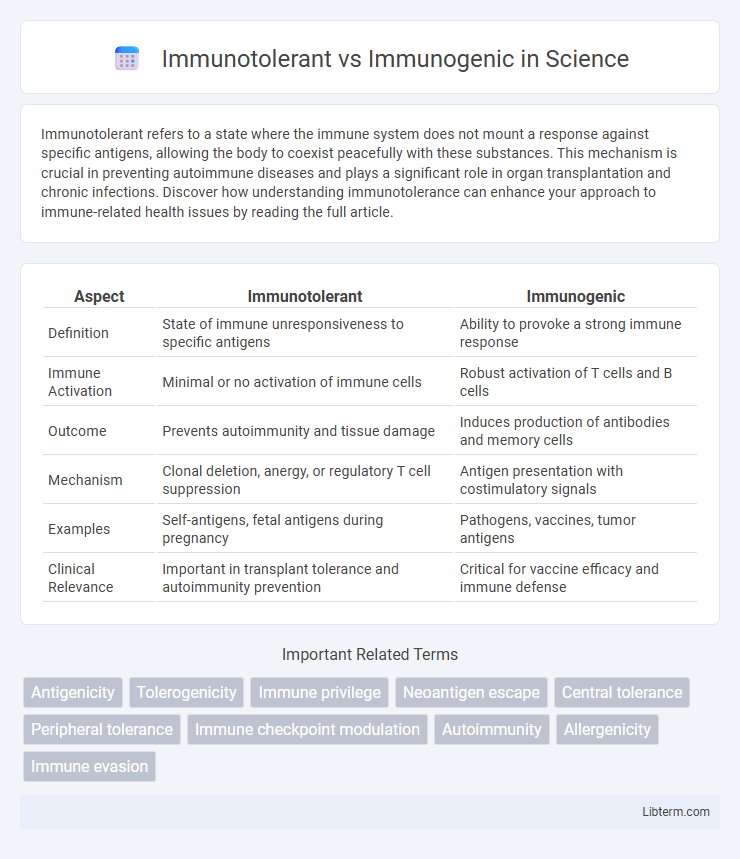
| Aspect | Immunotolerant | Immunogenic |
|---|---|---|
| Definition | State of immune unresponsiveness to specific antigens | Ability to provoke a strong immune response |
| Immune Activation | Minimal or no activation of immune cells | Robust activation of T cells and B cells |
| Outcome | Prevents autoimmunity and tissue damage | Induces production of antibodies and memory cells |
| Mechanism | Clonal deletion, anergy, or regulatory T cell suppression | Antigen presentation with costimulatory signals |
| Examples | Self-antigens, fetal antigens during pregnancy | Pathogens, vaccines, tumor antigens |
| Clinical Relevance | Important in transplant tolerance and autoimmunity prevention | Critical for vaccine efficacy and immune defense |
Introduction to Immunotolerance and Immunogenicity
Immunotolerance refers to the immune system's ability to recognize and avoid attacking the body's own tissues or harmless antigens, maintaining immune homeostasis and preventing autoimmune diseases. Immunogenicity describes the capacity of a substance, such as a pathogen or vaccine component, to provoke an immune response by activating immune cells and generating antibodies. Understanding the mechanisms behind immunotolerance and immunogenicity is essential for developing effective therapies and vaccines that balance immune protection and self-tolerance.
Defining Immunotolerant and Immunogenic Responses
Immunotolerant responses describe the immune system's ability to recognize and refrain from attacking self-antigens, maintaining homeostasis and preventing autoimmune diseases. Immunogenic responses involve the activation of immune cells against foreign antigens, leading to the production of antibodies and cytotoxic T cells to eliminate pathogens. Understanding the molecular mechanisms that distinguish immunotolerant from immunogenic responses is critical for advancing treatments in autoimmunity, transplantation, and vaccine development.
Mechanisms Underlying Immunotolerance
Immunotolerance involves complex mechanisms such as clonal anergy, regulatory T cell (Treg) induction, and deletion of autoreactive lymphocytes to prevent immune system activation against self-antigens. Central tolerance occurs in primary lymphoid organs like the thymus, where self-reactive T cells undergo negative selection, while peripheral tolerance employs mechanisms including immune checkpoint molecules (e.g., PD-1, CTLA-4) to suppress inappropriate immune responses. These processes contrast with immunogenic responses that actively stimulate antigen-specific T and B cell activation, cytokine production, and effector functions to eliminate pathogens or tumors.
Factors Influencing Immunogenicity
Immunogenicity is influenced by factors such as antigen size, molecular complexity, and dose, where larger and more complex molecules typically induce stronger immune responses than smaller, simpler ones. The route of antigen administration affects immune activation, with intramuscular and subcutaneous injections often eliciting more robust immunity compared to oral delivery, which tends to promote immunotolerance. Host factors including genetic background, age, and immune status also play critical roles in determining whether an antigen triggers immunogenicity or induces immunotolerance.
Immunotolerant vs Immunogenic: Key Differences
Immunotolerant refers to the immune system's ability to recognize and avoid attacking the body's own cells or harmless antigens, maintaining self-tolerance and preventing autoimmune diseases. Immunogenic describes substances or antigens that provoke a strong immune response, leading to the production of antibodies or activation of immune cells. Key differences include immunotolerance promoting immune suppression and self-recognition, while immunogenicity triggers active immune engagement and defense mechanisms.
Clinical Implications in Autoimmune Diseases
Immunotolerant states minimize immune system activation against self-antigens, reducing the risk of autoimmune tissue damage and improving patient outcomes in diseases such as type 1 diabetes and multiple sclerosis. Immunogenic responses trigger autoreactive T and B cells, leading to chronic inflammation and progressive organ dysfunction, necessitating aggressive immunosuppressive therapies. Understanding the balance between immunotolerance and immunogenicity guides targeted treatments, including tolerance-inducing biologics and antigen-specific immunotherapies, which can modulate disease progression and reduce adverse effects.
Role in Vaccine Development and Efficacy
Immunotolerant environments limit the immune system's response to vaccines, posing challenges in developing effective immunizations against certain pathogens or cancer cells. Immunogenic vaccines provoke a strong immune response, crucial for generating lasting immunity and high efficacy rates in preventing infections. Understanding and manipulating the balance between immunotolerance and immunogenicity enhances vaccine design, improving protective outcomes and minimizing adverse reactions.
Therapeutic Strategies Leveraging Immunotolerance
Therapeutic strategies leveraging immunotolerance aim to selectively suppress harmful immune responses while preserving overall immune function, targeting autoimmune diseases and transplant rejection. Techniques such as antigen-specific tolerance induction, regulatory T cell (Treg) enhancement, and immune checkpoint modulation promote immune homeostasis and minimize systemic immunosuppression. Advanced methods including nanoparticle delivery systems and gene editing tools like CRISPR are being developed to optimize precision in inducing long-lasting immunotolerance.
Challenges in Balancing Immunogenicity and Immunotolerance
Balancing immunogenicity and immunotolerance remains a significant challenge in immunotherapy and vaccine development due to the complex interplay between activating immune responses and preventing autoimmunity. High immunogenicity is essential for effective pathogen clearance or tumor eradication, yet excessive immune activation can lead to detrimental effects such as chronic inflammation or autoimmune diseases. Strategies that modulate antigen presentation, T-cell activation, and regulatory immune cells are critical to achieving precise control over immune tolerance without compromising protective immunity.
Future Perspectives in Immunomodulation
Immunotolerant therapies aim to induce long-lasting immune tolerance to prevent autoimmune reactions, representing a promising avenue for treating chronic inflammatory diseases with minimal side effects. Immunogenic approaches focus on enhancing immune responses, particularly in cancer immunotherapy, by activating specific T cells to target tumors effectively. Future perspectives in immunomodulation emphasize combining immunotolerant and immunogenic strategies to achieve precise control over immune activation and suppression, leveraging advances in personalized medicine and biomarker-driven treatments.
Immunotolerant Infographic
 libterm.com
libterm.com