Immunogenic substances trigger an immune response by stimulating the production of antibodies or activating immune cells. Understanding the mechanisms behind immunogenicity is crucial for vaccine development and autoimmune disease treatment. Explore the full article to learn how immunogenic factors can impact your health and therapies.
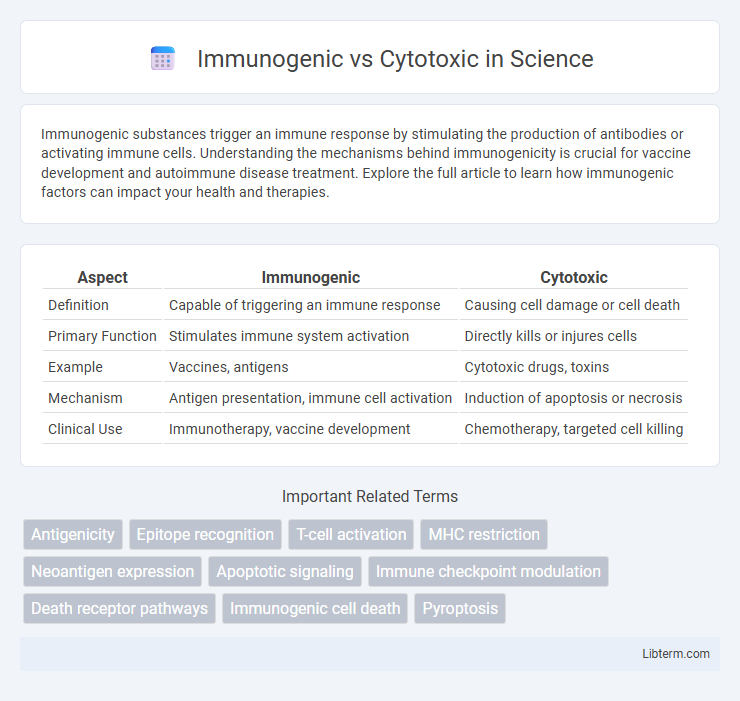
Table of Comparison
| Aspect | Immunogenic | Cytotoxic |
|---|---|---|
| Definition | Capable of triggering an immune response | Causing cell damage or cell death |
| Primary Function | Stimulates immune system activation | Directly kills or injures cells |
| Example | Vaccines, antigens | Cytotoxic drugs, toxins |
| Mechanism | Antigen presentation, immune cell activation | Induction of apoptosis or necrosis |
| Clinical Use | Immunotherapy, vaccine development | Chemotherapy, targeted cell killing |
Understanding Immunogenicity and Cytotoxicity
Immunogenicity refers to the ability of a substance, such as a vaccine or antigen, to provoke an immune response by activating immune cells and producing antibodies. Cytotoxicity, on the other hand, involves the capacity of certain cells or compounds to induce cell damage or death, often measured by assays evaluating cell viability or apoptosis. Understanding immunogenicity and cytotoxicity is crucial for developing safe and effective therapeutics, balancing immune activation with minimal harmful effects.
Key Differences Between Immunogenic and Cytotoxic Responses
Immunogenic responses activate the immune system by presenting antigens that stimulate adaptive immunity, leading to long-lasting protection and memory cell formation. Cytotoxic responses involve the direct killing of target cells, such as infected or cancerous cells, primarily through cytotoxic T lymphocytes and natural killer cells releasing perforin and granzymes. The key difference lies in immunogenicity promoting immune system activation and memory, while cytotoxicity focuses on immediate cell destruction without necessarily inducing long-term immunity.
Mechanisms of Immunogenic Cell Death
Immunogenic cell death (ICD) triggers a robust immune response by releasing danger-associated molecular patterns (DAMPs) such as calreticulin, ATP, and HMGB1, which enhance dendritic cell maturation and T-cell activation. Cytotoxic cell death, primarily executed through apoptosis or necrosis, causes direct cell destruction without necessarily inducing an adaptive immune response. The key distinction in ICD mechanisms lies in the active engagement of the immune system via antigen presentation and inflammatory signaling, promoting long-lasting antitumor immunity.
Mechanisms of Cytotoxicity in Cells
Mechanisms of cytotoxicity in cells involve the induction of programmed cell death through pathways such as apoptosis, necrosis, and autophagy, triggered by toxins, immune effector molecules, or chemotherapy agents. Cytotoxic agents disrupt cellular function by damaging DNA, inhibiting protein synthesis, or altering membrane integrity, leading to cell lysis or death. Immunogenic responses refer to the activation of the immune system by antigens, while cytotoxicity specifically describes the lethal effect on target cells, often mediated by cytotoxic T lymphocytes and natural killer cells releasing perforin and granzymes.
Role in Cancer Therapy: Immunogenic vs Cytotoxic Agents
Immunogenic agents in cancer therapy stimulate the immune system to recognize and attack tumor cells by promoting antigen presentation and activating T-cell responses. Cytotoxic agents directly kill cancer cells through mechanisms such as DNA damage, apoptosis induction, or cell cycle disruption, often affecting both cancerous and normal cells. Combining immunogenic and cytotoxic therapies enhances treatment efficacy by leveraging targeted immune activation alongside direct tumor cell eradication.
Immunogenicity in Vaccines and Immunotherapies
Immunogenicity in vaccines and immunotherapies refers to the ability to provoke a strong and specific immune response, crucial for long-lasting protection against pathogens or cancer cells. Unlike cytotoxic effects that directly kill cells, immunogenic approaches stimulate the body's immune system to recognize and target diseased cells, enhancing effectiveness and specificity. Optimizing immunogenic components such as adjuvants and epitopes increases vaccine efficacy and improves immune memory formation in therapeutic applications.
Cytotoxic Agents in Chemotherapy
Cytotoxic agents in chemotherapy function by directly killing rapidly dividing cancer cells through mechanisms such as DNA damage, inhibition of cell division, and induction of apoptosis. These agents include alkylating agents, antimetabolites, and topoisomerase inhibitors, which target specific phases of the cell cycle to maximize cancer cell death. Unlike immunogenic therapies that stimulate the immune response against tumors, cytotoxic chemotherapy relies on its cell-killing properties, often leading to side effects due to damage in healthy proliferating cells.
Evaluating Immunogenic and Cytotoxic Effects in Drug Development
Evaluating immunogenic and cytotoxic effects in drug development involves assessing the potential of drug candidates to trigger immune responses or cause cellular toxicity, which can impact safety and efficacy profiles. Immunogenicity testing includes analyzing antigen-antibody interactions, cytokine release assays, and T-cell activation, while cytotoxicity evaluation utilizes assays such as MTT, LDH release, and flow cytometry to measure cell viability and apoptosis. Integrating these assessments early in the preclinical phase helps optimize therapeutic dosing, reduce adverse effects, and improve clinical outcomes.
Measuring and Assessing Immunogenic vs Cytotoxic Reactions
Measuring immunogenic reactions involves evaluating the activation of the immune system through biomarkers like cytokine production, T-cell proliferation, and antibody generation using assays such as ELISA, flow cytometry, and ELISpot. Assessing cytotoxic reactions relies on detecting cell death or damage via lactate dehydrogenase (LDH) release, MTT assays, and Annexin V/PI staining to quantify apoptosis or necrosis levels. Combining these techniques provides comprehensive insights into the balance between immune activation and cellular toxicity in drug development and immunotherapy.
Future Directions in Immunogenic and Cytotoxic Research
Future directions in immunogenic and cytotoxic research emphasize the development of personalized cancer vaccines and targeted immunotherapies that enhance immune system recognition while minimizing off-target effects. Advanced techniques such as CRISPR gene editing and single-cell RNA sequencing enable precise modulation of immune responses and identification of novel biomarkers for improved therapeutic specificity. Integration of computational modeling with experimental validation accelerates the design of next-generation immunogenic agents and cytotoxic drugs, driving innovation in precision oncology.
Immunogenic Infographic
 libterm.com
libterm.com