Coordinate effective teamwork by aligning tasks, schedules, and communication to ensure seamless collaboration and project success. Proper coordination reduces errors and improves productivity, enabling your team to meet deadlines efficiently. Discover more strategies to optimize coordination in your projects by reading the rest of the article.
Table of Comparison
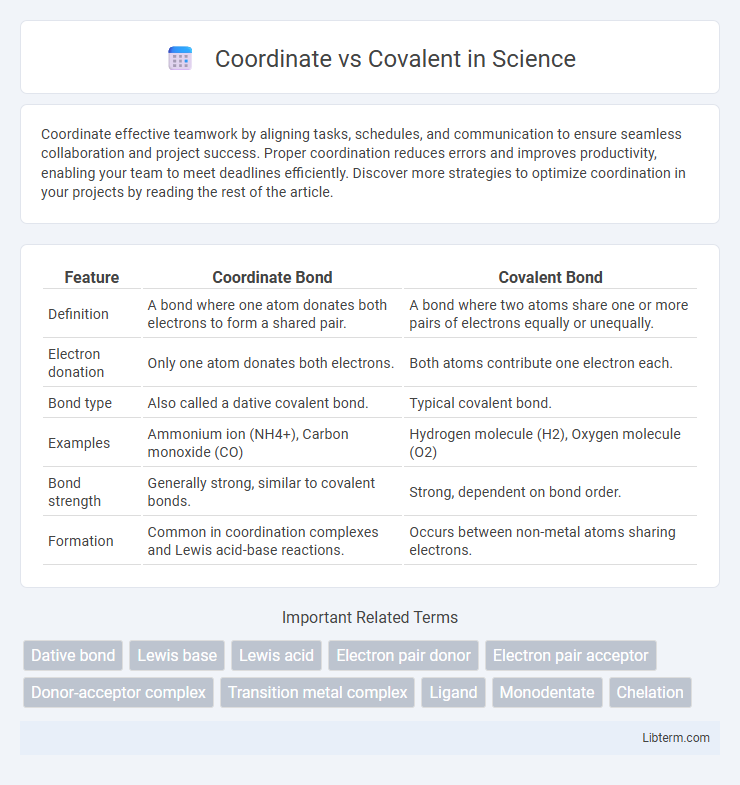
| Feature | Coordinate Bond | Covalent Bond |
|---|---|---|
| Definition | A bond where one atom donates both electrons to form a shared pair. | A bond where two atoms share one or more pairs of electrons equally or unequally. |
| Electron donation | Only one atom donates both electrons. | Both atoms contribute one electron each. |
| Bond type | Also called a dative covalent bond. | Typical covalent bond. |
| Examples | Ammonium ion (NH4+), Carbon monoxide (CO) | Hydrogen molecule (H2), Oxygen molecule (O2) |
| Bond strength | Generally strong, similar to covalent bonds. | Strong, dependent on bond order. |
| Formation | Common in coordination complexes and Lewis acid-base reactions. | Occurs between non-metal atoms sharing electrons. |
Introduction to Coordinate and Covalent Bonds
Coordinate bonds form when one atom donates both electrons in a shared pair, typically involving a Lewis base and a Lewis acid. Covalent bonds arise from the mutual sharing of electron pairs between atoms, leading to stable molecules by fulfilling octet configurations. Both bond types play essential roles in molecular structure and chemical reactivity, with coordinate bonds often observed in complex ions and coordination compounds.
Defining Coordinate Bonds
Coordinate bonds form when one atom donates both electrons to a shared pair, differing from covalent bonds where each atom contributes one electron. These bonds often occur between a Lewis base with a lone pair and a Lewis acid lacking electrons. Coordinate bonds are crucial in complex formation, such as metal-ligand interactions in coordination chemistry.
Understanding Covalent Bonds
Covalent bonds form when two atoms share one or more pairs of electrons, creating a strong chemical link that stabilizes molecules. These bonds typically occur between nonmetal atoms with similar electronegativities, resulting in shared electron density that holds the atoms together. Understanding covalent bonds is essential for explaining molecular structure, polarity, and chemical reactivity in compounds like water, carbon dioxide, and methane.
Key Differences Between Coordinate and Covalent Bonds
Coordinate bonds form when one atom donates both electrons to a shared pair, unlike covalent bonds where each atom contributes one electron. Coordinate bonds often occur in complexes involving transition metals, while covalent bonds are prevalent in organic and inorganic molecules. The electron sharing in coordinate bonds results in directional bonding, but the bond strength and length can differ significantly from typical covalent bonds.
Electron Sharing in Coordinate vs Covalent Bonds
Coordinate bonds involve one atom donating both electrons to form the bond, resulting in a shared electron pair originating from a single atom. In contrast, covalent bonds feature mutual electron sharing where each atom contributes one electron to the bond pair. The distinct electron donation in coordinate bonds leads to differences in bond polarity and reactivity compared to traditional covalent bonds.
Formation Mechanisms of Both Bond Types
Coordinate bonds form when one atom donates a lone pair of electrons to another atom that has an empty orbital, creating a shared electron pair exclusively contributed by one atom. Covalent bonds arise from the mutual sharing of electron pairs between two atoms, each contributing one electron to the bond. The key difference in formation mechanisms is that coordinate bonding involves a unilateral electron contribution, whereas covalent bonding involves bilateral electron sharing.
Examples of Coordinate Bonds in Chemistry
Coordinate bonds occur when one atom donates both electrons to form a shared pair, unlike covalent bonds where both atoms contribute electrons equally. Examples of coordinate bonds include the ammonium ion (NH4+), where the nitrogen atom donates a lone pair to a proton, and carbon monoxide (CO), where the carbon donates a lone pair to the metal in metal carbonyl complexes. Another common example is the bonding in complexes like the hexaamminecobalt(III) ion [Co(NH3)6]3+, where ammonia ligands donate lone pairs to the central cobalt ion.
Covalent Bond Examples in Everyday Life
Covalent bonds form when atoms share electron pairs, creating stable molecules commonly found in everyday substances like water (H2O), where hydrogen and oxygen atoms share electrons. Another example includes methane (CH4), the primary component of natural gas, where carbon shares electrons with four hydrogen atoms. These covalent interactions are essential in forming organic compounds, pharmaceuticals, and polymers such as nylon and polyester.
Importance and Applications in Modern Chemistry
Coordinate and covalent bonds play critical roles in modern chemistry, influencing molecular structure and reactivity. Coordinate bonds, where a single atom donates both electrons for bond formation, are essential in catalysis and bioinorganic chemistry, exemplified by metal complexes and enzyme active sites. Covalent bonds, involving shared electron pairs between atoms, form the backbone of organic molecules and materials science, enabling the design of pharmaceuticals, polymers, and nanomaterials.
Summary: Choosing Between Coordinate and Covalent Bonds
Coordinate bonds form when one atom donates both electrons to a shared pair, often seen in complex ions and coordination compounds, while covalent bonds involve equal sharing of electrons between atoms. Selecting between coordinate and covalent bonds depends on factors like electron availability, electronegativity differences, and molecular stability. Understanding their distinct bonding mechanisms aids in predicting compound behavior and reactivity in chemical and biological systems.
Coordinate Infographic
 libterm.com
libterm.com