Osmotic processes play a crucial role in regulating water movement across cell membranes, maintaining cellular balance and overall hydration. Understanding osmotic pressure and its effects can help you better appreciate its importance in biology and medical applications. Dive deeper into the article to explore how osmotic mechanisms influence health and technology.
Table of Comparison
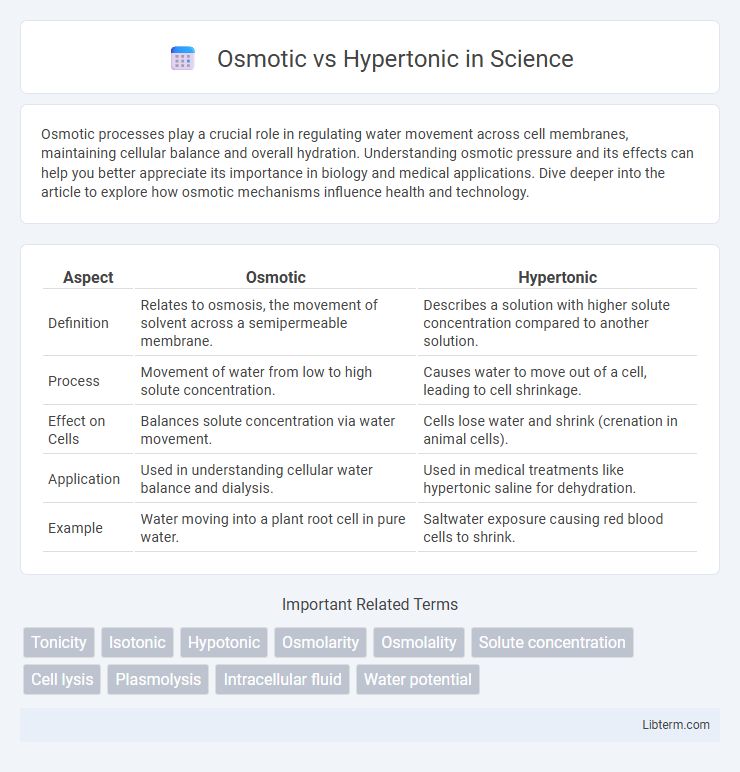
| Aspect | Osmotic | Hypertonic |
|---|---|---|
| Definition | Relates to osmosis, the movement of solvent across a semipermeable membrane. | Describes a solution with higher solute concentration compared to another solution. |
| Process | Movement of water from low to high solute concentration. | Causes water to move out of a cell, leading to cell shrinkage. |
| Effect on Cells | Balances solute concentration via water movement. | Cells lose water and shrink (crenation in animal cells). |
| Application | Used in understanding cellular water balance and dialysis. | Used in medical treatments like hypertonic saline for dehydration. |
| Example | Water moving into a plant root cell in pure water. | Saltwater exposure causing red blood cells to shrink. |
Introduction to Osmotic and Hypertonic Solutions
Osmotic solutions refer to fluids that balance solute concentration across membranes, allowing water to move naturally due to osmosis. Hypertonic solutions contain a higher concentration of solutes compared to the intracellular fluid, causing water to move out of cells and leading to cell shrinkage. Understanding the distinction between osmotic and hypertonic solutions is crucial in medical treatments like intravenous therapy and cellular hydration management.
Understanding Osmosis: The Basics
Osmosis is the passive movement of water molecules across a semi-permeable membrane from a region of lower solute concentration to a higher solute concentration, aiming to equalize solute levels on both sides. Hypertonic solutions have a higher solute concentration compared to the cell's interior, causing water to move out of the cell, leading to cell shrinkage. Understanding the osmotic gradient helps explain cellular behavior in different environments and is crucial for applications in medicine, biology, and chemistry.
What Defines a Hypertonic Solution?
A hypertonic solution is defined by having a higher concentration of solutes compared to the inside of a cell, causing water to move out of the cell by osmosis. This movement results in cell shrinkage or crenation due to water loss. The key characteristic of a hypertonic solution is its greater osmotic pressure relative to the intracellular environment.
Key Differences: Osmotic vs Hypertonic Effects
Osmotic effects refer to the movement of water across a semipermeable membrane driven by differences in solute concentration, maintaining cellular equilibrium. Hypertonic effects occur when extracellular fluid has a higher solute concentration than the intracellular fluid, causing water to move out of cells, leading to cell shrinkage. Understanding these differences is crucial in medical treatments like intravenous therapy and managing fluid balance.
Biological Impacts of Osmotic Pressure
Osmotic pressure regulates water movement across cell membranes, maintaining cellular homeostasis by balancing solute concentrations inside and outside the cell. Hypertonic solutions, with higher solute concentrations than the cell's interior, cause water to exit the cell, leading to shrinkage and impaired cellular function. This mechanism is critical in processes such as kidney function, where osmotic gradients drive water reabsorption and maintain fluid balance in tissues.
Hypertonic Solutions in Medical Applications
Hypertonic solutions have a higher concentration of solutes compared to the intracellular fluid, causing water to move out of cells by osmosis. In medical applications, hypertonic saline (e.g., 3% NaCl) is commonly used to reduce cerebral edema and treat hyponatremia by drawing excess fluid from swollen brain tissue into the bloodstream. These solutions require careful monitoring to prevent dehydration, electrolyte imbalance, and vascular complications.
Comparative Table: Osmotic vs Hypertonic Solutions
Osmotic and hypertonic solutions differ primarily in their solute concentration relative to a cell's interior; osmotic solutions regulate water movement to maintain cell volume, while hypertonic solutions have higher solute concentrations causing water to exit the cell, leading to cell shrinkage. Osmotic solutions are essential in maintaining cellular homeostasis and fluid balance, whereas hypertonic solutions are often used clinically to reduce cerebral edema or treat hyponatremia by drawing water out of cells. Key parameters distinguishing these solutions include osmolarity, tonicity, and their physiological impact on cells, with osmotic solutions being isotonic or slightly hypotonic, and hypertonic solutions exceeding normal extracellular solute levels.
Real-World Examples in Nature and Industry
Osmotic processes drive water movement across cell membranes, exemplified by plant roots absorbing water from soil, while hypertonic solutions, such as saline used in medical treatments, cause cells to shrink by drawing out water. In industry, osmotic principles enable desalination through reverse osmosis membranes, whereas hypertonic brine solutions play a critical role in food preservation by inhibiting microbial growth. Understanding the distinctions between osmotic and hypertonic effects allows optimization in agriculture, healthcare, and food processing applications.
Common Misconceptions and Clarifications
Osmotic solutions refer broadly to any solution that influences water movement across a semipermeable membrane, whereas hypertonic solutions specifically have a higher solute concentration compared to the cell's interior, causing water to exit the cell. A common misconception is that osmotic and hypertonic solutions are interchangeable terms, but osmotic encompasses isotonic, hypertonic, and hypotonic conditions influencing cellular fluid balance differently. Clarifying these distinctions is critical in clinical settings where the precise tonicity of intravenous fluids impacts patient hydration and cell integrity.
Conclusion: Choosing the Right Solution Type
Selecting between osmotic and hypertonic solutions depends on the desired medical outcome and patient condition; osmotic solutions effectively balance fluid levels by promoting water movement across membranes, while hypertonic solutions draw water out of cells to reduce swelling or edema. Understanding the specific cellular response to each solution type ensures optimal therapeutic results and minimizes potential complications. Accurate assessment of patient status and treatment goals is crucial for choosing the appropriate solution.
Osmotic Infographic
 libterm.com
libterm.com