Amidation is a critical biochemical process where an amine group is introduced into a molecule, often enhancing its stability and activity in pharmaceutical compounds. This reaction plays a pivotal role in the synthesis of peptides, drugs, and various organic compounds by forming amide bonds that contribute to molecular functionality. Explore this article to understand how amidation influences your chemical synthesis and its applications across industries.
Table of Comparison
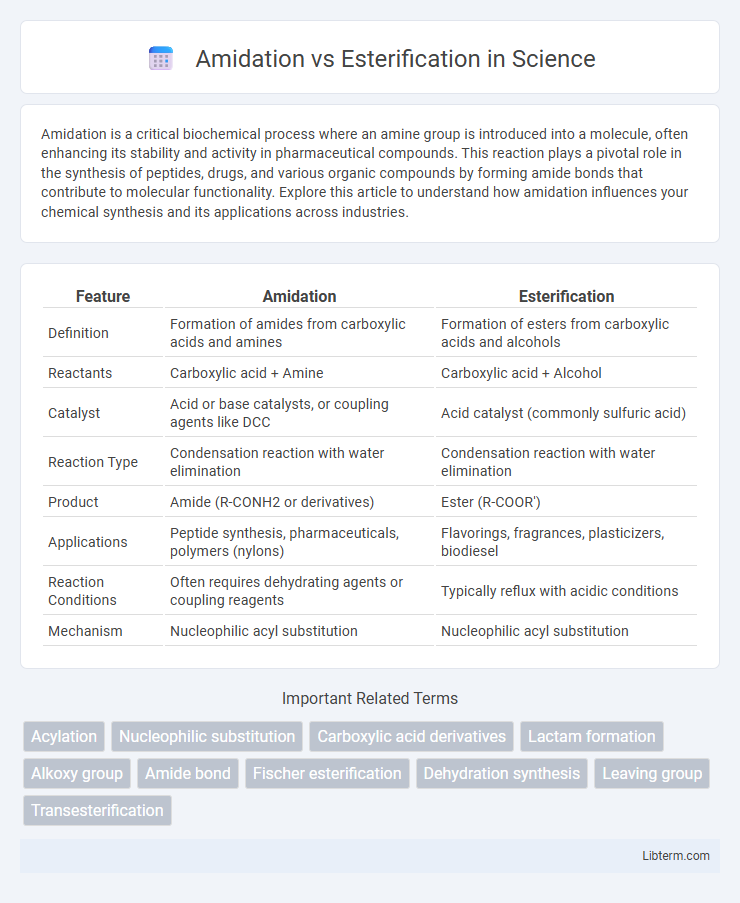
| Feature | Amidation | Esterification |
|---|---|---|
| Definition | Formation of amides from carboxylic acids and amines | Formation of esters from carboxylic acids and alcohols |
| Reactants | Carboxylic acid + Amine | Carboxylic acid + Alcohol |
| Catalyst | Acid or base catalysts, or coupling agents like DCC | Acid catalyst (commonly sulfuric acid) |
| Reaction Type | Condensation reaction with water elimination | Condensation reaction with water elimination |
| Product | Amide (R-CONH2 or derivatives) | Ester (R-COOR') |
| Applications | Peptide synthesis, pharmaceuticals, polymers (nylons) | Flavorings, fragrances, plasticizers, biodiesel |
| Reaction Conditions | Often requires dehydrating agents or coupling reagents | Typically reflux with acidic conditions |
| Mechanism | Nucleophilic acyl substitution | Nucleophilic acyl substitution |
Introduction to Amidation and Esterification
Amidation and esterification are fundamental chemical reactions used to form amides and esters, respectively, by combining carboxylic acids with amines or alcohols. Amidation involves the formation of an amide bond through the reaction of a carboxylic acid group with an amine, producing water as a byproduct, commonly utilized in peptide synthesis and polymer production. Esterification refers to the reaction between a carboxylic acid and an alcohol, catalyzed by acids or enzymes, leading to the formation of esters important in fragrance, flavor industries, and biodegradable plastics.
Fundamental Concepts: Amidation Explained
Amidation is a fundamental organic reaction where a carboxylic acid or its derivative reacts with an amine to form an amide, characterized by a carbonyl group bonded to a nitrogen atom. This process involves nucleophilic attack by the amine on the electrophilic carbonyl carbon, followed by the elimination of water or another leaving group, resulting in strong amide bonds crucial for protein structure and synthetic polymers. Unlike esterification, which forms esters via reaction of carboxylic acids with alcohols, amidation produces more stable amide linkages due to resonance stabilization and lower hydrolysis rates.
Fundamental Concepts: Esterification Explained
Esterification is a fundamental organic reaction where a carboxylic acid reacts with an alcohol, producing an ester and water through a condensation mechanism. This process typically requires an acid catalyst, such as sulfuric acid, to enhance the reaction rate by protonating the carbonyl oxygen of the acid. Esterification plays a crucial role in synthesizing esters, which are important in manufacturing fragrances, solvents, and polymers.
Reaction Mechanisms: Amidation vs. Esterification
Amidation involves nucleophilic attack of an amine on a carbonyl carbon of a carboxylic acid or its derivative, followed by elimination of water or a leaving group, forming an amide bond. Esterification typically proceeds via nucleophilic attack of an alcohol on a protonated carboxylic acid, leading to ester formation through a tetrahedral intermediate and dehydration. Both reaction mechanisms proceed through nucleophilic acyl substitution but differ in the nucleophile and intermediate stability, affecting reaction conditions and rates.
Key Differences in Chemical Structures
Amidation involves the formation of an amide bond between a carboxylic acid and an amine, resulting in a functional group characterized by a carbonyl group (C=O) directly attached to a nitrogen atom (N). Esterification, on the other hand, produces an ester linkage through the reaction of a carboxylic acid with an alcohol, featuring a carbonyl group bonded to an oxygen atom (O) connected to an alkyl or aryl group. The fundamental structural difference lies in the presence of a nitrogen atom in amides versus an oxygen atom in esters, significantly affecting their chemical properties and reactivity.
Catalysts and Reaction Conditions
Amidation typically requires strong acid or base catalysts such as sulfuric acid or metal oxides to activate the carbonyl group under elevated temperatures, often ranging from 150degC to 250degC. Esterification commonly employs acid catalysts like sulfuric acid or Lewis acids such as zinc chloride, with reaction conditions including reflux temperatures around 60degC to 130degC and removal of water to drive equilibrium. Both reactions benefit from catalysts that increase electrophilicity of carboxylic acids, but amidation generally demands harsher thermal conditions and stronger catalytic systems due to the lower nucleophilicity of amines versus alcohols.
Industrial Applications and Significance
Amidation and esterification are critical reactions in pharmaceutical and polymer industries, enabling the synthesis of amides and esters that serve as key intermediates and final products. Amidation is widely used in producing pharmaceuticals such as antibiotics, peptides, and agrochemicals due to the stability and bioactivity of amide bonds. Esterification finds extensive application in manufacturing plasticizers, fragrances, and biodiesel, leveraging ester compounds' versatility and biodegradability.
Efficiency and Yield Comparison
Amidation reactions generally achieve higher efficiency and yield compared to esterification due to the stronger nucleophilicity of amines over alcohols, enabling faster bond formation with carboxylic acids or their derivatives. Esterification often requires acidic conditions and longer reaction times, which can reduce overall yield and increase side product formation. Industrial processes favor amidation when targeting amide products, as optimized catalysts and solvents further enhance their reaction rates and selectivity, resulting in superior throughput and purity.
Environmental and Safety Considerations
Amidation typically generates fewer volatile organic compounds (VOCs) compared to esterification, reducing air pollution and health risks in industrial settings. Esterification often involves strong acids or bases and flammable alcohols, increasing the risk of hazardous spills and fire. Selecting amidation processes with green catalysts and milder conditions enhances safety and lowers environmental impact.
Future Trends and Innovations
Future trends in amidation and esterification emphasize green chemistry approaches, such as enzyme-catalyzed reactions and solvent-free processes, enhancing sustainability and reducing environmental impact. Innovations include the development of selective catalysts enabling milder reaction conditions and higher efficiency in pharmaceutical and polymer synthesis. Advances in continuous flow reactors and biocatalysts are driving scalable and economically viable production methods for amidation and esterification reactions.
Amidation Infographic
 libterm.com
libterm.com