Electrolytes are substances that conduct electricity when dissolved in water by releasing ions, crucial for processes like battery operation and biological functions. Insulators, on the other hand, prevent the flow of electric current by lacking free charge carriers, making them essential for protecting circuits and ensuring safety. Explore the rest of the article to understand how both play vital roles in electrical and electronic applications.
Table of Comparison
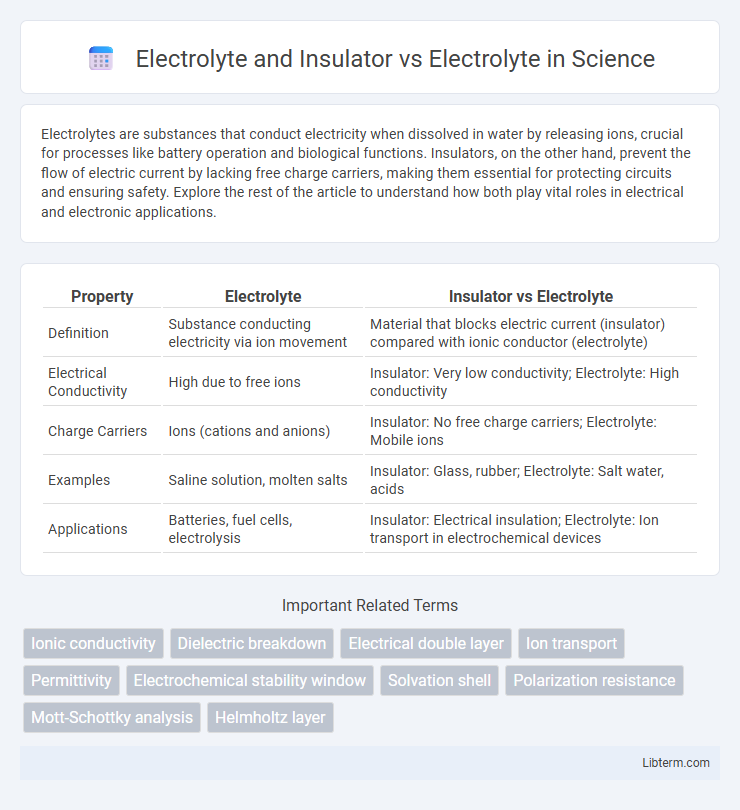
| Property | Electrolyte | Insulator vs Electrolyte |
|---|---|---|
| Definition | Substance conducting electricity via ion movement | Material that blocks electric current (insulator) compared with ionic conductor (electrolyte) |
| Electrical Conductivity | High due to free ions | Insulator: Very low conductivity; Electrolyte: High conductivity |
| Charge Carriers | Ions (cations and anions) | Insulator: No free charge carriers; Electrolyte: Mobile ions |
| Examples | Saline solution, molten salts | Insulator: Glass, rubber; Electrolyte: Salt water, acids |
| Applications | Batteries, fuel cells, electrolysis | Insulator: Electrical insulation; Electrolyte: Ion transport in electrochemical devices |
Understanding Electrolytes: Definition and Properties
Electrolytes are substances that dissociate into ions when dissolved in a solvent, enabling the conduction of electricity, whereas insulators prevent electrical conduction due to the absence of free ions or electrons. Key properties of electrolytes include ionization ability, conductivity, and mobility of ions in solutions. Understanding these properties is essential for applications in batteries, biological systems, and chemical processes.
What Are Insulators? Key Characteristics
Insulators are materials that resist the flow of electric current due to their tightly bound electrons, making them essential for preventing unwanted conduction in electrical systems. Key characteristics of insulators include high electrical resistance, low conductivity, and the ability to block or significantly reduce the movement of free ions or electrons. Unlike electrolytes, which conduct electricity through ion movement in solutions, insulators inhibit electron mobility and maintain circuit integrity by isolating conductive components.
Electrolyte vs Insulator: Fundamental Differences
Electrolytes are substances that dissociate into ions and conduct electricity through ionic movement, essential for processes like nerve signaling and battery function. Insulators, in contrast, are materials that resist electrical conduction due to the absence of free charge carriers, typically used to prevent current flow and protect circuits. The fundamental difference lies in electrolytes enabling ionic conductivity while insulators restrict electrical charge movement.
Types of Electrolytes: Strong vs Weak
Electrolytes are substances that dissociate into ions in solution, enabling electrical conductivity. Strong electrolytes, such as sodium chloride and hydrochloric acid, completely ionize in aqueous solutions, providing high conductivity. Weak electrolytes like acetic acid and ammonia partially dissociate, resulting in lower ion concentration and reduced conductivity, distinguishing them from insulators which do not allow ion flow.
Physical and Chemical Behaviors of Electrolytes
Electrolytes are substances that dissociate into ions and conduct electricity in aqueous solutions, exhibiting high ionic conductivity and characteristic colligative properties. Unlike insulators, electrolytes facilitate charge transfer through ion mobility, influenced by factors such as concentration, temperature, and solvent polarity. Their chemical behavior involves ionization, dissociation equilibria, and interactions with solvents, which determine conductivity, conductivity behavior, and reactivity in electrochemical processes.
Examples of Common Electrolytes and Insulators
Common electrolytes include sodium chloride, potassium chloride, and calcium chloride, which dissociate into ions and conduct electricity in aqueous solutions. Insulators such as rubber, glass, and plastic do not conduct electric current due to the absence of free ions or electrons. Electrolytes play vital roles in biological systems and industrial processes, while insulators are essential for preventing electrical conduction and ensuring safety in electronic devices.
Electrical Conductivity in Electrolytes and Insulators
Electrolytes exhibit high electrical conductivity due to the presence of free ions that facilitate the flow of electric current, making them essential in batteries and electrochemical cells. In contrast, insulators lack free charge carriers, resulting in extremely low electrical conductivity as their electrons are tightly bound and unable to move freely. The fundamental difference in electrical conductivity between electrolytes and insulators stems from ion mobility in electrolytes versus the absence of mobile charges in insulators.
Applications: Electrolytes in Batteries vs Insulators in Circuits
Electrolytes in batteries facilitate ion conduction, enabling efficient energy storage and transfer critical for devices ranging from smartphones to electric vehicles. Insulators in circuits prevent unwanted current flow, ensuring component protection and maintaining signal integrity in electronic devices and power systems. The distinct roles of electrolytes and insulators optimize performance in energy storage and electronic circuitry applications.
Importance of Electrolytes and Insulators in Daily Life
Electrolytes are essential for maintaining fluid balance, nerve function, and muscular activity by conducting electric currents in biological systems, while insulators prevent the unwanted flow of electricity, ensuring safety and functionality in electronic devices and household wiring. Understanding the distinct roles of electrolytes in bodily functions and insulators in protecting circuits highlights their critical importance for health and technological infrastructure. Proper electrolyte balance supports hydration and cellular processes, whereas effective insulation reduces the risk of electrical hazards and energy loss in daily applications.
Future Trends in Electrolyte and Insulator Technologies
Future trends in electrolyte and insulator technologies emphasize the development of solid-state electrolytes to enhance battery safety and energy density, replacing traditional liquid electrolytes prone to leakage and flammability. Advanced ceramic and polymer-based insulators are being engineered to improve ionic conductivity while maintaining electric insulation, critical for next-generation energy storage and electronic devices. Innovations include nano-engineered materials and hybrid composites that aim to optimize interface stability, mechanical flexibility, and thermal management in applications such as solid-state batteries and flexible electronics.
Electrolyte and Insulator Infographic
 libterm.com
libterm.com