Mass spectrometry is a powerful analytical technique used to identify the composition and structure of molecules by measuring the mass-to-charge ratio of ions. This method provides detailed insights into molecular weight, chemical properties, and molecular interactions, making it essential in fields like chemistry, biology, and pharmacology. Explore the rest of the article to understand how mass spectrometry can enhance your analytical capabilities.
Table of Comparison
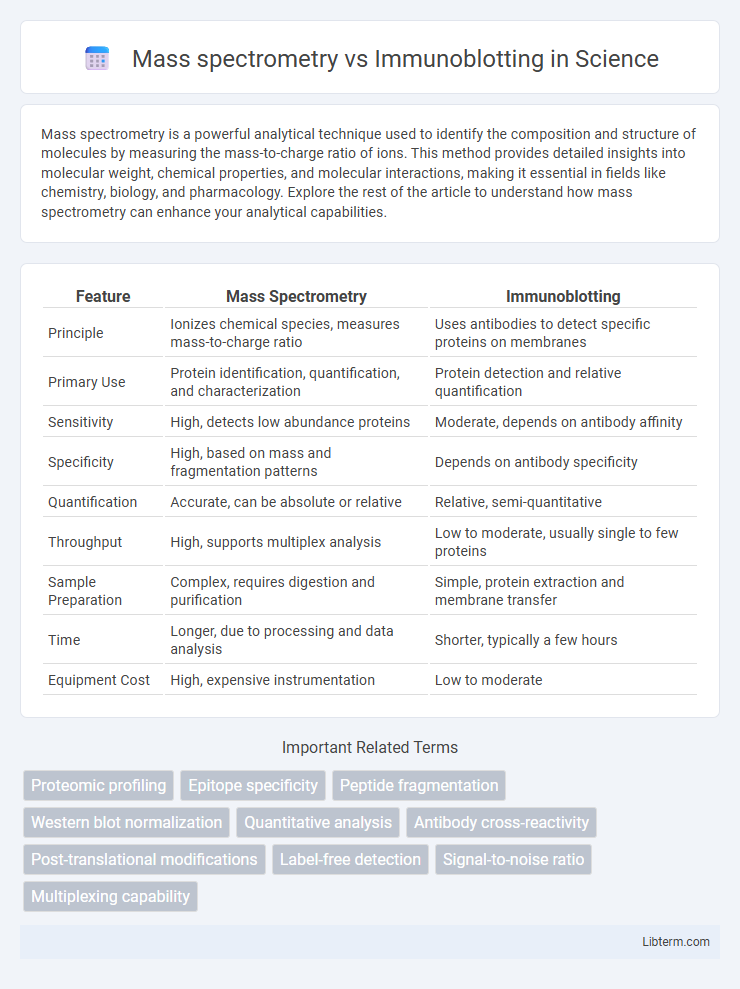
| Feature | Mass Spectrometry | Immunoblotting |
|---|---|---|
| Principle | Ionizes chemical species, measures mass-to-charge ratio | Uses antibodies to detect specific proteins on membranes |
| Primary Use | Protein identification, quantification, and characterization | Protein detection and relative quantification |
| Sensitivity | High, detects low abundance proteins | Moderate, depends on antibody affinity |
| Specificity | High, based on mass and fragmentation patterns | Depends on antibody specificity |
| Quantification | Accurate, can be absolute or relative | Relative, semi-quantitative |
| Throughput | High, supports multiplex analysis | Low to moderate, usually single to few proteins |
| Sample Preparation | Complex, requires digestion and purification | Simple, protein extraction and membrane transfer |
| Time | Longer, due to processing and data analysis | Shorter, typically a few hours |
| Equipment Cost | High, expensive instrumentation | Low to moderate |
Overview of Mass Spectrometry and Immunoblotting
Mass spectrometry offers high-throughput, precise identification and quantification of proteins by measuring mass-to-charge ratios of ionized particles, enabling detailed proteomic analysis. Immunoblotting, also known as Western blotting, detects specific proteins through antibody binding, providing qualitative and semi-quantitative data on protein expression. Both methods are essential in molecular biology, with mass spectrometry excelling in comprehensive protein profiling and immunoblotting specializing in targeted protein validation.
Principles and Mechanisms of Mass Spectrometry
Mass spectrometry operates by ionizing chemical compounds to generate charged molecules or molecular fragments and measuring their mass-to-charge ratios using electromagnetic fields. This technique provides precise molecular weight determination and structural information based on the fragmentation patterns of analytes. Unlike immunoblotting, which relies on antibody-antigen interactions for detection, mass spectrometry offers high sensitivity and specificity through direct mass analysis without the need for labeled antibodies.
Fundamentals of Immunoblotting Techniques
Immunoblotting techniques, also known as Western blotting, rely on antibody-antigen interactions to detect specific proteins within a complex mixture, using gel electrophoresis followed by membrane transfer. This fundamental method involves separating proteins based on molecular weight, immobilizing them on a membrane, and probing with primary and secondary antibodies conjugated to enzymatic or fluorescent reporters for visualization. Unlike mass spectrometry, which identifies proteins through mass-to-charge ratio analysis and peptide fragmentation, immunoblotting provides qualitative and semi-quantitative data crucial for protein expression and post-translational modification studies.
Sample Preparation: Requirements and Challenges
Mass spectrometry requires precise sample preparation including protein extraction, digestion into peptides, and often desalting to enhance ionization efficiency, which can be time-consuming and demands technical expertise. Immunoblotting involves protein denaturation and separation by gel electrophoresis, followed by transfer to membranes and antibody probing, presenting challenges such as maintaining protein integrity and preventing non-specific binding. Both techniques face difficulties in handling complex biological samples and require careful optimization to ensure accurate, reproducible results.
Sensitivity and Specificity Comparison
Mass spectrometry offers superior sensitivity by detecting low-abundance proteins with high accuracy through precise mass-to-charge ratio measurements, enabling identification of minute molecular differences. Immunoblotting provides high specificity using antibody-antigen interactions but often suffers from lower sensitivity due to limitations in antibody affinity and detection methods. While mass spectrometry excels in quantifying proteins across complex mixtures, immunoblotting remains valuable for confirming protein presence when specific antibodies are available.
Quantitative and Qualitative Analysis Capabilities
Mass spectrometry offers highly sensitive quantitative analysis by measuring precise molecular masses and abundances of proteins and peptides, enabling identification of post-translational modifications and protein isoforms with high specificity. Immunoblotting provides qualitative detection of target proteins through antigen-antibody interactions, allowing visualization of protein presence and relative abundance but with limited quantification accuracy. Mass spectrometry excels in comprehensive proteomic profiling, while immunoblotting remains valuable for validating protein expression and detecting specific targets in complex samples.
Applications in Proteomics and Diagnostics
Mass spectrometry offers high-throughput, precise protein identification and quantification, making it essential for comprehensive proteomic studies and biomarker discovery in diagnostics. Immunoblotting provides targeted protein detection and validation with high specificity, useful for confirming protein expression and post-translational modifications in clinical samples. Combining mass spectrometry's broad profiling capabilities with immunoblotting's confirmatory analysis enhances accuracy and depth in proteomic research and diagnostic applications.
Advantages of Mass Spectrometry
Mass spectrometry offers unparalleled sensitivity and specificity in protein identification and quantification, enabling the detection of thousands of proteins simultaneously within complex mixtures. It provides precise molecular weight determination and post-translational modification analysis, which immunoblotting lacks due to its dependence on antibody specificity. High-throughput capacity and quantitative accuracy make mass spectrometry an essential tool for proteomics and biomarker discovery compared to the semi-quantitative, antibody-reliant nature of immunoblotting.
Strengths of Immunoblotting
Immunoblotting offers high specificity by using antibodies to detect target proteins within complex mixtures, enabling precise protein identification and quantification. It provides visual confirmation of protein size through gel electrophoresis, allowing detection of post-translational modifications and protein isoforms. The technique is cost-effective and accessible, making it ideal for validating protein expression and studying protein-protein interactions in various biological samples.
Choosing Between Mass Spectrometry and Immunoblotting: Key Considerations
Choosing between mass spectrometry and immunoblotting depends on the specific research goals, such as the need for protein identification, quantification, or post-translational modification analysis. Mass spectrometry offers high sensitivity and multiplexing capabilities, enabling detailed protein characterization and discovery-based studies, while immunoblotting provides targeted detection with high specificity for known proteins. Consider sample complexity, required sensitivity, throughput, and available resources when deciding the most appropriate technique for proteomic analysis.
Mass spectrometry Infographic
 libterm.com
libterm.com