Necrosis is the uncontrolled death of cells caused by injury, infection, or insufficient blood supply, leading to tissue damage and inflammation. Understanding the types and causes of necrosis can help in diagnosing and managing various medical conditions effectively. Explore the article to learn how necrosis impacts your health and the available treatment options.
Table of Comparison
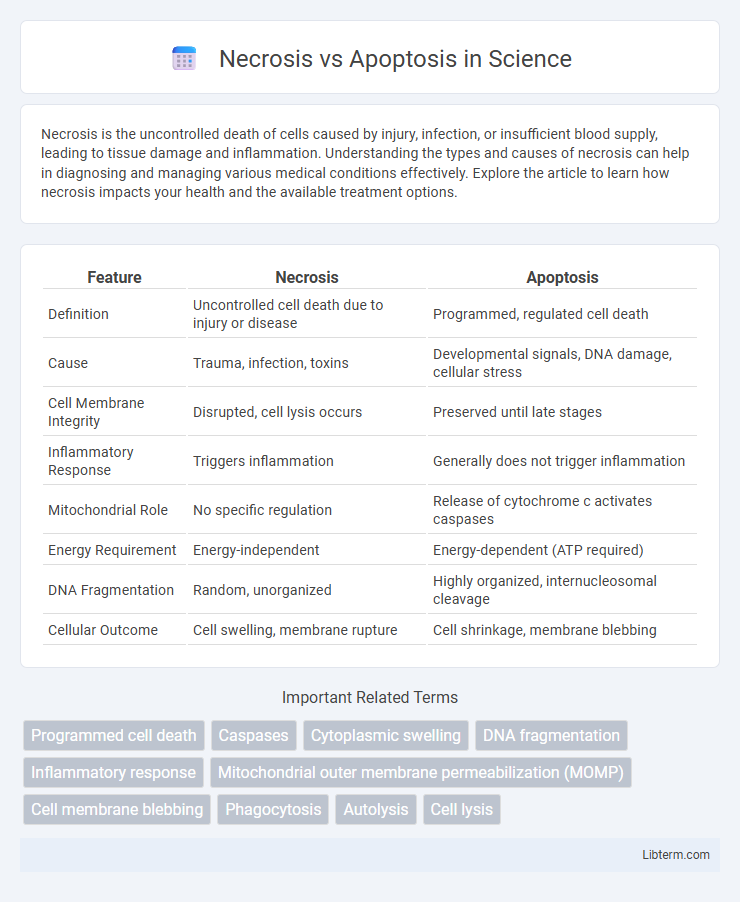
| Feature | Necrosis | Apoptosis |
|---|---|---|
| Definition | Uncontrolled cell death due to injury or disease | Programmed, regulated cell death |
| Cause | Trauma, infection, toxins | Developmental signals, DNA damage, cellular stress |
| Cell Membrane Integrity | Disrupted, cell lysis occurs | Preserved until late stages |
| Inflammatory Response | Triggers inflammation | Generally does not trigger inflammation |
| Mitochondrial Role | No specific regulation | Release of cytochrome c activates caspases |
| Energy Requirement | Energy-independent | Energy-dependent (ATP required) |
| DNA Fragmentation | Random, unorganized | Highly organized, internucleosomal cleavage |
| Cellular Outcome | Cell swelling, membrane rupture | Cell shrinkage, membrane blebbing |
Introduction to Cell Death: Necrosis vs Apoptosis
Cell death mechanisms are primarily categorized into necrosis and apoptosis, each with distinct cellular processes and physiological outcomes. Necrosis is an uncontrolled, pathological process resulting from acute cellular injury, leading to membrane rupture and inflammation. Apoptosis, in contrast, is a regulated, programmed cell death essential for maintaining tissue homeostasis and eliminating damaged cells without eliciting an inflammatory response.
Defining Necrosis: Key Features and Processes
Necrosis is a form of uncontrolled cell death characterized by the loss of membrane integrity, swelling of organelles, and subsequent cell lysis, often triggering an inflammatory response. Key features include mitochondrial dysfunction, the release of intracellular contents such as proteolytic enzymes and damage-associated molecular patterns (DAMPs), and disruption of tissue homeostasis. Necrosis typically results from acute cellular injury caused by factors like ischemia, toxins, or trauma, leading to energy depletion and irreversible cell damage.
Understanding Apoptosis: Hallmarks and Mechanisms
Apoptosis is characterized by distinct morphological changes such as cell shrinkage, chromatin condensation, and membrane blebbing, driven by caspase activation and mitochondrial cytochrome c release. Key mechanisms involve intrinsic and extrinsic pathways that regulate programmed cell death through a balance of pro-apoptotic and anti-apoptotic Bcl-2 family proteins. This tightly controlled process ensures tissue homeostasis and prevents inflammation, contrasting with necrosis, which results from uncontrolled cell injury and triggers inflammatory responses.
Molecular Pathways: Necrosis and Apoptosis Compared
Necrosis is characterized by uncontrolled cell death involving the loss of membrane integrity and release of cellular contents through pathways such as receptor-interacting protein kinase 1 (RIPK1) and RIPK3-mediated necroptosis. Apoptosis proceeds via well-defined molecular pathways, including the intrinsic mitochondrial pathway regulated by Bcl-2 family proteins and cytochrome c release, and the extrinsic death receptor pathway activating caspases like caspase-8 and caspase-3. These distinct molecular cascades delineate apoptosis as a programmed, energy-dependent process, whereas necrosis often results from acute cellular injury causing passive and inflammatory cell demise.
Triggers and Causes: What Initiates Each Process?
Necrosis is primarily triggered by external factors such as physical injury, toxins, infection, or ischemia, leading to the uncontrollable death of cells due to severe damage. Apoptosis is initiated by intrinsic signals like DNA damage, oxidative stress, or developmental cues, as well as extrinsic signals from death ligands binding to cell surface receptors, systematically activating programmed cell death pathways. Both processes serve distinct biological roles, with necrosis causing inflammation and apoptosis maintaining tissue homeostasis through regulated cell elimination.
Morphological Differences: Necrosis vs Apoptosis
Necrosis is characterized by cell swelling, plasma membrane rupture, and the subsequent release of intracellular contents, leading to inflammation. In contrast, apoptosis involves cell shrinkage, chromatin condensation, nuclear fragmentation, and formation of apoptotic bodies that are phagocytosed without triggering inflammation. These morphological distinctions are critical for identifying the mode of cell death in pathological and physiological contexts.
Physiological and Pathological Roles
Necrosis primarily results from acute cellular injury causing uncontrolled cell death, leading to inflammation and tissue damage, playing a pathological role in conditions such as ischemia, trauma, and infection. Apoptosis is a regulated process of programmed cell death essential for maintaining tissue homeostasis, immune system function, and embryonic development, with physiological roles in removing damaged or unneeded cells. Dysregulation of apoptosis contributes to pathological states including cancer, autoimmune diseases, and neurodegenerative disorders by either excessive cell survival or unwarranted cell loss.
Implications in Disease: Necrosis and Apoptosis in Health
Necrosis involves uncontrolled cell death leading to inflammation and tissue damage, commonly seen in conditions such as ischemic injury, infections, and trauma. Apoptosis is a programmed, regulated cell death crucial for maintaining cellular homeostasis and preventing cancer by eliminating damaged cells. Dysregulation of apoptosis can contribute to autoimmune diseases, neurodegeneration, and tumor progression, highlighting its essential role in health and disease management.
Detection Methods: Identifying Cell Death Types
Necrosis and apoptosis are identified through distinct detection methods, with necrosis commonly detected via propidium iodide staining that marks compromised cell membranes, while apoptosis is frequently identified using Annexin V binding assays detecting phosphatidylserine externalization. Flow cytometry and fluorescence microscopy enhance accuracy in distinguishing these cell death modalities by analyzing specific biomarkers such as caspase activation for apoptosis and lactate dehydrogenase release for necrosis. Advanced techniques like TUNEL assay target DNA fragmentation characteristic of apoptosis, providing a comprehensive toolkit for precise cell death type analysis.
Therapeutic Approaches Targeting Necrosis and Apoptosis
Therapeutic approaches targeting necrosis focus on inhibiting the uncontrolled cell death pathways by using necrostatins and anti-inflammatory agents to prevent tissue damage in ischemic injuries. Apoptosis-based therapies leverage molecules like BH3 mimetics and caspase inhibitors to selectively induce or block programmed cell death in cancer and neurodegenerative diseases. Modulating signaling pathways such as RIPK1/RIPK3 for necrosis and Bcl-2 family proteins for apoptosis enhances treatment specificity and minimizes adverse effects.
Necrosis Infographic
 libterm.com
libterm.com