Electrolytes are substances that dissociate into ions when dissolved in water, allowing the solution to conduct electricity, while nonelectrolytes do not dissociate and thus do not conduct electricity. Understanding the difference between electrolytes and nonelectrolytes is crucial for applications in chemistry, biology, and medicine, especially in maintaining your body's fluid balance and nerve function. Explore the full article to learn more about their properties, examples, and significance.
Table of Comparison
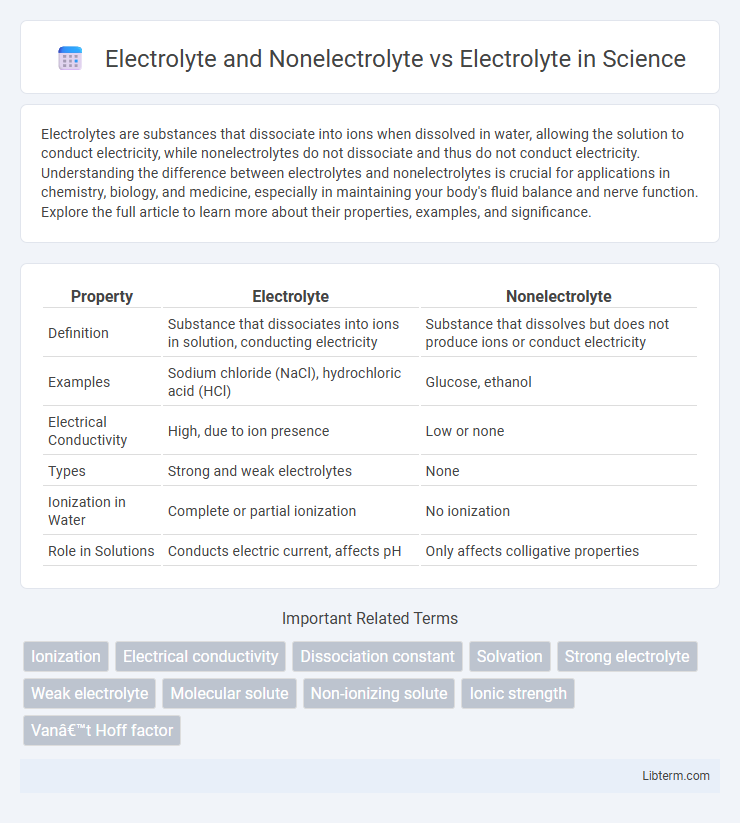
| Property | Electrolyte | Nonelectrolyte |
|---|---|---|
| Definition | Substance that dissociates into ions in solution, conducting electricity | Substance that dissolves but does not produce ions or conduct electricity |
| Examples | Sodium chloride (NaCl), hydrochloric acid (HCl) | Glucose, ethanol |
| Electrical Conductivity | High, due to ion presence | Low or none |
| Types | Strong and weak electrolytes | None |
| Ionization in Water | Complete or partial ionization | No ionization |
| Role in Solutions | Conducts electric current, affects pH | Only affects colligative properties |
Introduction to Electrolytes and Nonelectrolytes
Electrolytes are substances that dissociate into ions when dissolved in water, enabling the solution to conduct electricity, such as sodium chloride and potassium chloride. Nonelectrolytes, like glucose and urea, dissolve in water without producing ions, resulting in poor or no electrical conductivity. Understanding the distinction between electrolytes and nonelectrolytes is essential for applications in biochemistry, medicine, and industrial processes involving ionic conduction.
Defining Electrolytes: Properties and Types
Electrolytes are substances that dissociate into ions when dissolved in water, enabling the solution to conduct electricity, characterized by properties such as ionic conductivity and degree of ionization. Unlike nonelectrolytes, which dissolve without forming ions and do not conduct electricity, electrolytes are classified into strong electrolytes, fully dissociating in solution, and weak electrolytes, partially ionizing and producing fewer ions. Common examples include salts like sodium chloride (strong electrolyte) and acetic acid (weak electrolyte), essential for physiological functions and industrial processes.
What Are Nonelectrolytes? Characteristics and Examples
Nonelectrolytes are substances that do not dissociate into ions when dissolved in water, resulting in no electrical conductivity. Characteristically, nonelectrolytes include compounds like glucose, urea, and ethanol, which dissolve as intact molecules rather than ions. These properties distinguish nonelectrolytes from electrolytes, which ionize in solution and conduct electricity.
Key Differences: Electrolyte vs Nonelectrolyte
Electrolytes are substances that dissociate into ions when dissolved in water, conducting electricity, whereas nonelectrolytes do not dissociate and therefore do not conduct electricity. Examples of electrolytes include sodium chloride and potassium hydroxide, while glucose and urea are common nonelectrolytes. The key difference lies in ionization in solution, which determines their electrical conductivity.
How Electrolytes Conduct Electricity
Electrolytes are substances that dissociate into ions when dissolved in water, enabling the solution to conduct electricity efficiently due to the movement of free ions. Nonelectrolytes, by contrast, do not dissociate into ions and thus do not conduct electricity in solution. The conductivity in electrolytes depends on factors such as ion concentration, ion mobility, and the degree of ionization of the dissolved salt, acid, or base.
Nonelectrolytes and Electrical Conductivity
Nonelectrolytes are substances that do not dissociate into ions in solution, resulting in negligible electrical conductivity. Unlike electrolytes, which ionize and enhance a solution's ability to conduct electricity, nonelectrolytes such as glucose or ethanol remain molecularly intact and thus do not contribute free charge carriers. Their presence in a solution maintains low conductivity, making them essential in applications requiring non-conductive media.
Common Examples of Electrolytes
Common examples of electrolytes include sodium chloride (NaCl), potassium chloride (KCl), calcium chloride (CaCl2), and magnesium sulfate (MgSO4), which dissociate into ions in aqueous solutions to conduct electricity. Nonelectrolytes such as sugar or ethanol do not ionize and therefore do not conduct electricity in solution. Electrolytes are essential in biological systems for maintaining nerve function, muscle contraction, and fluid balance through their ionic activity.
Everyday Nonelectrolyte Substances
Everyday nonelectrolyte substances such as sugar, ethanol, and urea dissolve in water without dissociating into ions, resulting in no electrical conductivity. Unlike electrolytes like sodium chloride and potassium hydroxide, which ionize and conduct electricity, nonelectrolytes retain molecular integrity in solution. This fundamental difference influences applications in biological systems, food chemistry, and industrial processes where electrical conductivity and ion availability are critical.
Practical Applications: Electrolytes in Real Life
Electrolytes such as sodium chloride and potassium chloride play crucial roles in maintaining hydration and nerve function in medical treatments like intravenous therapy and sports drinks. Nonelectrolytes, including sugar and alcohol, do not ionize and thus lack electrical conductivity, limiting their use in applications requiring electrical charge transfer. Electrolytes are essential in batteries, industrial processes, and biological systems due to their ability to dissociate into ions, enabling electrical conductivity and chemical reactivity.
Comparative Summary: Electrolyte vs Nonelectrolyte
Electrolytes dissociate into ions in solution, enabling electrical conductivity, whereas nonelectrolytes do not ionize and thus do not conduct electricity. Strong electrolytes like sodium chloride fully dissociate, while weak electrolytes such as acetic acid partially ionize, contrasting with nonelectrolytes like sugar that remain intact. The conductivity difference is critical in applications ranging from biological systems to industrial processes, highlighting the distinct ionic behavior of electrolytes versus the molecular stability of nonelectrolytes.
Electrolyte and Nonelectrolyte Infographic
 libterm.com
libterm.com