Diamagnetic materials generate an induced magnetic field in the opposite direction to an applied magnetic field, causing them to be repelled by magnets. These materials, including copper, bismuth, and graphite, exhibit weak magnetic properties due to their electron configurations, which produce no permanent magnetic moment. Explore the rest of the article to understand how diamagnetism contrasts with other magnetic behaviors and its practical implications for your applications.
Table of Comparison
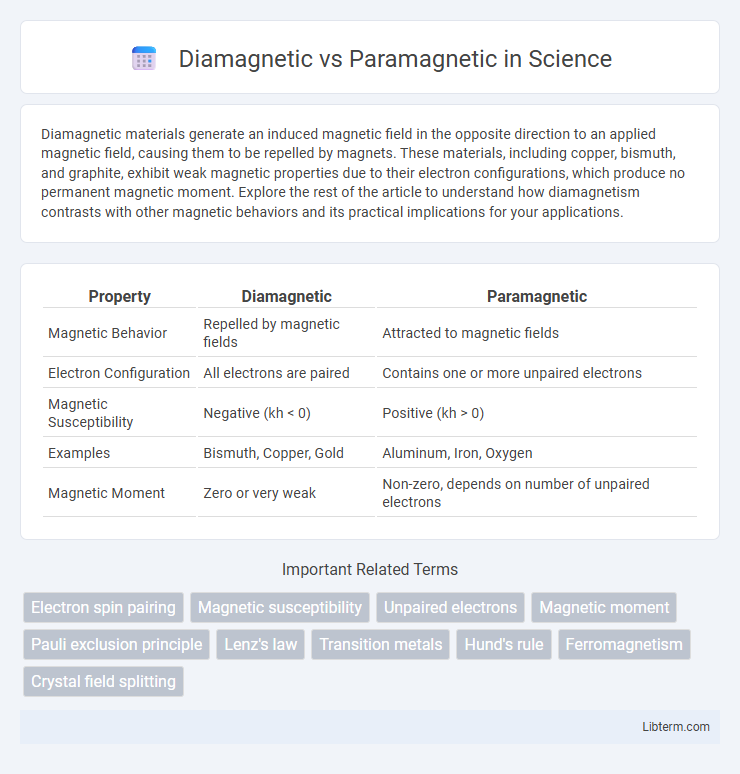
| Property | Diamagnetic | Paramagnetic |
|---|---|---|
| Magnetic Behavior | Repelled by magnetic fields | Attracted to magnetic fields |
| Electron Configuration | All electrons are paired | Contains one or more unpaired electrons |
| Magnetic Susceptibility | Negative (kh < 0) | Positive (kh > 0) |
| Examples | Bismuth, Copper, Gold | Aluminum, Iron, Oxygen |
| Magnetic Moment | Zero or very weak | Non-zero, depends on number of unpaired electrons |
Introduction to Magnetic Properties
Diamagnetic materials create an induced magnetic field in the opposite direction to an applied external magnetic field, resulting in a weak repulsion due to paired electrons with no net magnetic moment. Paramagnetic materials contain unpaired electrons that align with the external magnetic field, producing a weak attraction and a positive magnetic susceptibility. These fundamental distinctions in electron configuration determine the intrinsic magnetic responses critical to applications in materials science and magnetic sensing technologies.
What is Diamagnetism?
Diamagnetism is a fundamental property of all materials characterized by the creation of an induced magnetic field in a direction opposite to an externally applied magnetic field. This phenomenon arises from the orbital motion of paired electrons generating tiny current loops that oppose the change in magnetic flux. Materials such as bismuth, copper, and silver exhibit diamagnetism, which is generally weak and only observable in the absence of stronger paramagnetic or ferromagnetic effects.
What is Paramagnetism?
Paramagnetism refers to the property of materials that have unpaired electrons, resulting in a net magnetic moment that aligns with an external magnetic field, causing attraction. Paramagnetic substances, such as aluminum and oxygen, exhibit weak magnetic susceptibility and only retain magnetization while exposed to the magnetic field. This contrasts with diamagnetic materials, which have all electrons paired and create an induced magnetic field in the opposite direction, leading to weak repulsion.
Key Differences Between Diamagnetic and Paramagnetic Materials
Diamagnetic materials exhibit no net magnetic moment as all their electrons are paired, resulting in a weak repulsion from magnetic fields. Paramagnetic materials contain one or more unpaired electrons, generating a net magnetic moment that causes a weak attraction to external magnetic fields. The magnetic susceptibility of diamagnetic substances is negative, while paramagnetic substances display positive magnetic susceptibility.
Electron Configurations and Magnetism
Diamagnetic materials have all their electrons paired, resulting in no net magnetic moment and weak repulsion from magnetic fields. Paramagnetic materials contain at least one unpaired electron, producing a net magnetic moment that causes attraction to magnetic fields. Electron configurations determine magnetic properties by the presence or absence of unpaired electrons in atomic or molecular orbitals.
Examples of Diamagnetic Substances
Diamagnetic substances, such as bismuth, copper, and zinc, exhibit no unpaired electrons and are repelled by magnetic fields due to their paired electron configurations. These materials generate an induced magnetic field in the opposite direction when exposed to an external magnetic field, resulting in weak magnetic repulsion. Common examples like helium, nitrogen, and silver also demonstrate diamagnetism, making them essential in studies of magnetic susceptibilities and applications requiring materials with minimal magnetic interference.
Examples of Paramagnetic Substances
Paramagnetic substances contain unpaired electrons, causing them to be attracted to magnetic fields, with classic examples including oxygen (O2), iron (Fe3+), and manganese (Mn2+). Transition metal ions like chromium (Cr3+), copper (Cu2+), and nickel (Ni2+) also exhibit paramagnetism due to their unpaired d-electrons. These materials contrast with diamagnetic substances, which have all electrons paired and are weakly repelled by magnetic fields.
Applications of Diamagnetic and Paramagnetic Materials
Diamagnetic materials find applications in magnetic levitation and magnetic resonance imaging (MRI) due to their ability to create weak repulsive magnetic fields, which stabilize floating objects and improve image contrast. Paramagnetic materials are widely used in catalysts, sensors, and oxygen transport systems because of their unpaired electrons that enhance magnetic susceptibility and chemical reactivity. Both types of materials play crucial roles in quantum computing and spintronics, where control over magnetic properties enables advanced data storage and processing technologies.
Experimental Methods to Identify Magnetism
Experimental methods to identify diamagnetic and paramagnetic materials include vibrating sample magnetometry (VSM) and SQUID magnetometry, which measure magnetic susceptibility and magnetization response to applied magnetic fields. Electron spin resonance (ESR) spectroscopy detects unpaired electrons, characteristic of paramagnetic substances, while diamagnetic materials show no ESR signal due to the absence of unpaired spins. Magnetic susceptibility measurements using Gouy or Faraday balances quantitatively distinguish diamagnetism (negative susceptibility) from paramagnetism (positive susceptibility).
Summary: Choosing Between Diamagnetic and Paramagnetic
Diamagnetic materials exhibit no unpaired electrons, causing them to slightly repel magnetic fields, whereas paramagnetic materials contain unpaired electrons that align with magnetic fields, producing attraction. Selecting between diamagnetic and paramagnetic substances depends on their electron configurations and desired magnetic response in applications such as magnetic resonance imaging (MRI) contrast agents or magnetic separation processes. Understanding their magnetic susceptibilities aids in optimizing material choice for sensors, catalysts, and electronic devices requiring specific magnetic behaviors.
Diamagnetic Infographic
 libterm.com
libterm.com