Allomorphism refers to the phenomenon where a single morpheme manifests in different forms depending on its phonological or morphological context. Understanding allomorphism is crucial for analyzing language patterns and improving linguistic accuracy in interpretation. Explore the article to uncover how allomorphs influence language structure and usage.
Table of Comparison
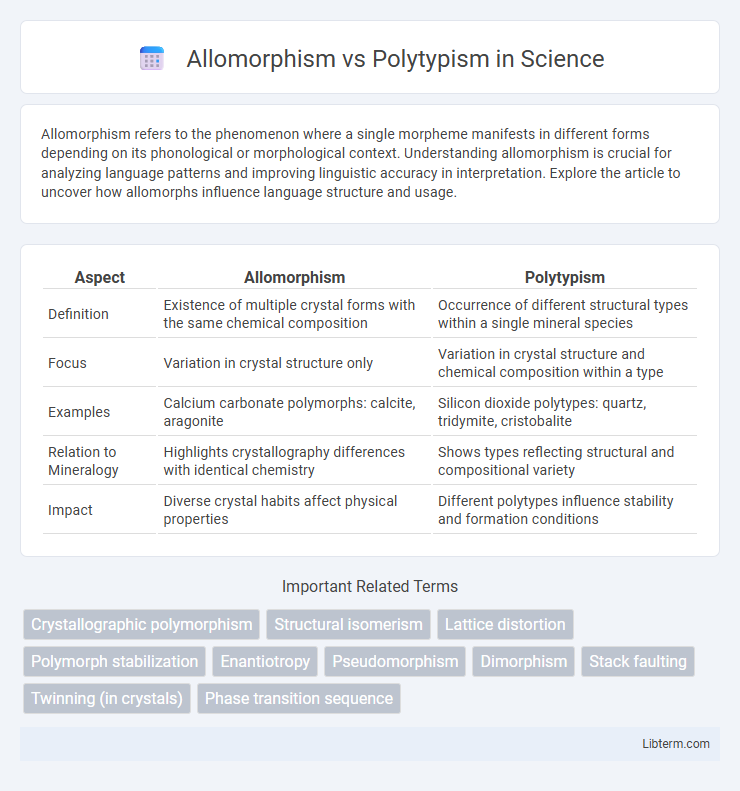
| Aspect | Allomorphism | Polytypism |
|---|---|---|
| Definition | Existence of multiple crystal forms with the same chemical composition | Occurrence of different structural types within a single mineral species |
| Focus | Variation in crystal structure only | Variation in crystal structure and chemical composition within a type |
| Examples | Calcium carbonate polymorphs: calcite, aragonite | Silicon dioxide polytypes: quartz, tridymite, cristobalite |
| Relation to Mineralogy | Highlights crystallography differences with identical chemistry | Shows types reflecting structural and compositional variety |
| Impact | Diverse crystal habits affect physical properties | Different polytypes influence stability and formation conditions |
Understanding Allomorphism: Definition and Basics
Allomorphism refers to the phenomenon where a single morpheme exhibits different phonetic or morphological forms without changing its underlying meaning, such as the plural morpheme in English appearing as /s/, /z/, or /Iz/. It is essential to distinguish allomorphism from polytypism, which involves variations in word forms across different dialects or languages. Understanding allomorphism aids in analyzing morphological patterns and phonological rules within a language's structure.
Exploring Polytypism: What Does It Mean?
Polytypism refers to the capability of a programming language or system to define functions or data types that can operate uniformly across a variety of types, enabling code reuse and abstraction by focusing on structural similarities rather than specific type instances. Unlike allomorphism, which involves variations in form or structure, polytypism leverages type polymorphism to allow generic programming through type-driven design patterns, particularly in functional programming languages like Haskell. Understanding polytypism is essential for developers aiming to implement algorithms that adapt seamlessly to different data representations while maintaining type safety and reducing code duplication.
Allomorphism vs Polytypism: Key Differences
Allomorphism refers to variations of a morpheme that occur due to phonological or morphological contexts, resulting in different phonetic forms without changing meaning, whereas polytypism involves the existence of multiple related types or forms within a species or category, often used in biological taxonomy. The key difference lies in allomorphism's linguistic focus on morpheme variations versus polytypism's emphasis on categorization based on structural or genetic diversity. Understanding allomorphism aids in morphological analysis, while polytypism is crucial for studying species variation and classification.
Structural Variations in Crystals: An Overview
Allomorphism and polytypism represent distinct structural variations in crystals characterized by differing atomic arrangements; allomorphism involves multiple crystalline forms of the same substance with different chemical bonding patterns, while polytypism describes variations exclusively in the stacking sequences of identical two-dimensional layers. These phenomena influence physical properties such as hardness, density, and optical behavior due to changes in symmetry and lattice parameters. Understanding these structural differences is crucial for materials science applications, including semiconductor design and mineral identification.
Chemical Composition: Similarities and Contrasts
Allomorphism and polytypism both refer to variations in chemical compounds where the chemical composition remains constant while the crystal structure differs, affecting physical properties. In allomorphism, substances exhibit different crystal forms due to variations in environmental factors during formation, whereas polytypism involves stacking sequence variations within the same crystal structure type. Both phenomena demonstrate how identical chemical compositions can produce distinct structural modifications, influencing material characteristics such as hardness, solubility, and optical properties.
Real-world Examples of Allomorphism
Allomorphism is exemplified in geology by minerals such as diamond and graphite, which share the same chemical composition (carbon) but differ in crystal structure, leading to distinct physical properties. Another real-world example is the polymorphs of calcium carbonate: calcite and aragonite exhibit different crystalline forms despite identical chemical formulas. This contrasts with polytypism seen in layered materials like silicon carbide, where structural variations occur due to different stacking sequences rather than entirely distinct crystal systems.
Notable Cases of Polytypism in Minerals
Polytypism in minerals refers to the phenomenon where a compound exists in several structural forms differing only in the stacking sequence of identical layers, with classic examples including silicon carbide (SiC) and talc. Unlike allomorphism, which involves distinct chemical compositions and structures such as diamond versus graphite, polytypism maintains the same chemical formula while exhibiting variations in physical properties due to layer arrangement. Notable polytypic minerals demonstrate variations in stacking sequences that influence hardness, optical behavior, and crystallographic symmetry, essential for industrial applications and geological classification.
Implications for Material Properties
Allomorphism, referring to the existence of different crystal structures for the same chemical composition, significantly influences material properties such as hardness, melting point, and electrical conductivity due to variations in atomic arrangement. Polytypism, a specific form of polymorphism characterized by layer stacking variations, affects properties like optical behavior, mechanical strength, and thermal stability by altering interlayer bonding and symmetry. Understanding these structural differences enables tailored design of materials for specific industrial applications, including semiconductors, ceramics, and catalysts.
Analytical Techniques for Identification
Allomorphism and polytypism are identified using advanced analytical techniques such as X-ray diffraction (XRD) and Raman spectroscopy, which reveal structural variations at the atomic level. Differential scanning calorimetry (DSC) aids in distinguishing thermal behavior unique to each polymorph or allomorph. Complementary methods like infrared spectroscopy (IR) and solid-state nuclear magnetic resonance (NMR) provide detailed molecular environment information critical for accurate classification.
Importance in Materials Science and Industry
Allomorphism and polytypism significantly influence the physical properties and performance of materials in industries such as metallurgy, ceramics, and polymers. Understanding allomorphism, the existence of different crystal structures for the same chemical composition, enables the design of materials with tailored hardness, conductivity, and thermal stability. Polytypism, the variation in stacking sequences within layered structures, critically affects the electronic and mechanical behavior of materials like silicon carbide and transition metal dichalcogenides, enhancing their application in semiconductors and nanotechnology.
Allomorphism Infographic
 libterm.com
libterm.com