Nonmagnetic materials do not generate a magnetic field and are unaffected by external magnetic forces, making them essential in various industrial applications where magnetic interference must be avoided. These materials include substances like copper, plastic, and wood, widely used in electronics, medical devices, and precision instruments to ensure accurate performance. Discover more about the properties and uses of nonmagnetic materials in the rest of this article.
Table of Comparison
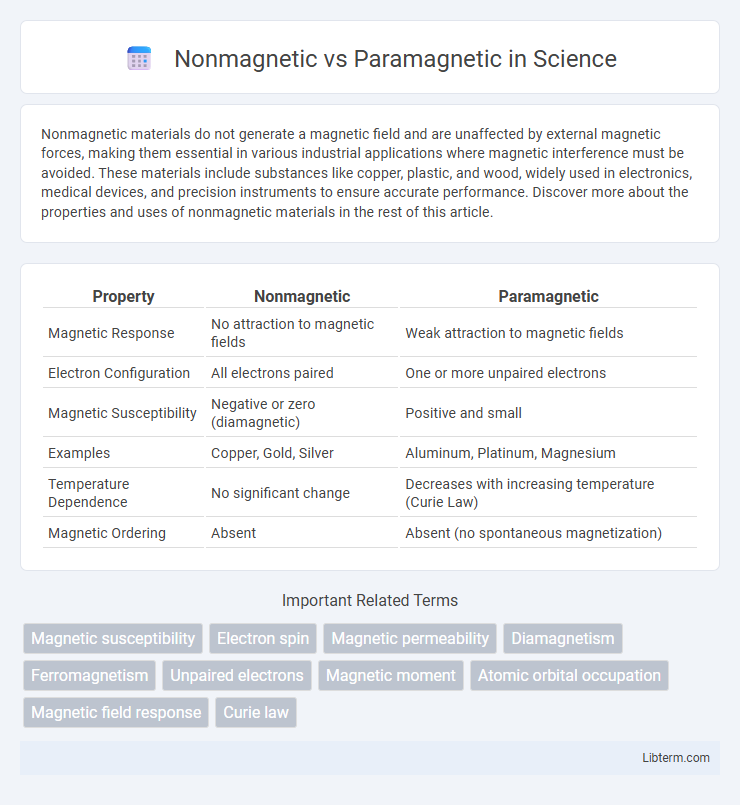
| Property | Nonmagnetic | Paramagnetic |
|---|---|---|
| Magnetic Response | No attraction to magnetic fields | Weak attraction to magnetic fields |
| Electron Configuration | All electrons paired | One or more unpaired electrons |
| Magnetic Susceptibility | Negative or zero (diamagnetic) | Positive and small |
| Examples | Copper, Gold, Silver | Aluminum, Platinum, Magnesium |
| Temperature Dependence | No significant change | Decreases with increasing temperature (Curie Law) |
| Magnetic Ordering | Absent | Absent (no spontaneous magnetization) |
Introduction to Magnetic Properties
Nonmagnetic materials exhibit no intrinsic magnetic moment and do not respond to external magnetic fields, remaining essentially unaffected by magnetic forces. Paramagnetic substances contain unpaired electrons, resulting in a weak attraction to external magnetic fields due to induced magnetic moments aligning with the field direction. The distinction between nonmagnetic and paramagnetic materials lies in their electronic structure and magnetic susceptibility, which determines their differing interactions with magnetic environments.
Defining Nonmagnetic Materials
Nonmagnetic materials are characterized by the absence of permanent magnetic moments in their atomic or molecular structure, meaning they do not exhibit magnetization when exposed to an external magnetic field. Unlike paramagnetic materials, which have unpaired electrons that cause temporary attraction to magnetic fields, nonmagnetic materials have all electrons paired, resulting in no net magnetic susceptibility. Common examples include copper, gold, and silver, which remain unaffected by magnetic forces, making them essential in applications requiring minimal magnetic interference.
Understanding Paramagnetic Materials
Paramagnetic materials contain unpaired electrons that align with external magnetic fields, resulting in a weak attraction. Unlike nonmagnetic substances, which lack unpaired electrons and show no magnetic response, paramagnetic materials exhibit positive magnetic susceptibility. Common examples include aluminum, platinum, and oxygen, which demonstrate how the electron configuration influences magnetic properties.
Atomic and Molecular Structure Differences
Nonmagnetic materials have atomic or molecular structures with paired electrons that create no net magnetic moment, causing them to be unaffected by external magnetic fields. Paramagnetic substances possess unpaired electrons in their atomic or molecular orbitals, generating a weak but positive magnetic susceptibility due to the alignment of these magnetic moments with an applied field. The difference in electron spin configurations primarily governs the magnetic properties, with paramagnetic atoms or molecules containing partially filled orbitals while nonmagnetic ones feature fully paired electron shells.
Magnetic Susceptibility: Nonmagnetic vs Paramagnetic
Nonmagnetic materials exhibit zero or negative magnetic susceptibility, indicating they are not attracted to magnetic fields and often create an opposing field when exposed to magnetism. Paramagnetic materials have a positive magnetic susceptibility, causing them to be weakly attracted to external magnetic fields due to unpaired electrons aligning with the field. The difference in magnetic susceptibility between nonmagnetic and paramagnetic substances is critical in applications such as magnetic resonance imaging (MRI) and magnetic separation techniques.
Common Examples and Applications
Nonmagnetic materials such as copper, aluminum, and gold do not exhibit magnetic attraction and are widely used in electrical wiring, aircraft, and electronic devices due to their excellent conductivity and corrosion resistance. Paramagnetic substances like aluminum, platinum, and manganese exhibit weak magnetic attraction only in the presence of an external magnetic field, making them suitable for applications in magnetic resonance imaging (MRI), catalysis, and sensors. The distinction between these materials is crucial for industries requiring precise control of magnetic properties to optimize performance and safety.
Influence of Temperature on Magnetism
Temperature significantly affects the magnetic properties of nonmagnetic and paramagnetic materials by influencing electron spin alignment. Nonmagnetic substances remain largely unaffected by temperature changes as they lack unpaired electrons, resulting in negligible magnetic susceptibility. Paramagnetic materials exhibit increased magnetic susceptibility at lower temperatures due to enhanced alignment of unpaired electron spins, while higher temperatures cause thermal agitation that disrupts this alignment, reducing magnetization.
Testing and Identifying Magnetic Types
Nonmagnetic materials exhibit no attraction to magnetic fields, making them easily identifiable through the absence of magnetic pull during testing with a magnet or a Gauss meter. Paramagnetic materials show weak attraction to magnetic fields and can be detected using sensitive instruments like a vibrating sample magnetometer (VSM) or SQUID magnetometer, which measure their slight magnetic susceptibility. Precise identification relies on these testing methods, differentiating nonmagnetic substances by zero magnetic response and paramagnetic materials by measurable, though minimal, positive magnetic susceptibility.
Practical Implications in Technology
Nonmagnetic materials, such as copper and aluminum, are crucial in electronic applications where interference from magnetic fields must be minimized, including in wiring and circuit boards. Paramagnetic materials like aluminum and platinum exhibit weak attraction to magnetic fields, enabling their use in magnetic resonance imaging (MRI) and sensors that detect subtle magnetic changes. Understanding the magnetic properties of these materials enhances device performance, durability, and accuracy in technologies ranging from medical imaging to aerospace engineering.
Conclusion: Choosing the Right Material
Nonmagnetic materials exhibit no intrinsic magnetic properties and remain unaffected by external magnetic fields, making them ideal for applications requiring electromagnetic interference shielding or corrosion resistance. Paramagnetic materials have unpaired electrons causing a weak attraction to magnetic fields, suitable for sensors and catalysts needing magnetic responsiveness without permanent magnetization. Selecting the right material depends on the specific application requirements, such as the need for magnetic sensitivity versus complete magnetic neutrality.
Nonmagnetic Infographic
 libterm.com
libterm.com