A racemate consists of equal amounts of two enantiomers, molecules that are non-superimposable mirror images of each other but share identical physical properties except for the direction they rotate plane-polarized light. These mixtures often arise in chemical syntheses where both enantiomers form in equal proportions, influencing drug efficacy and safety due to differing biological activities. Explore the article to understand how racemates impact pharmacology and methods for separating enantiomers in your applications.
Table of Comparison
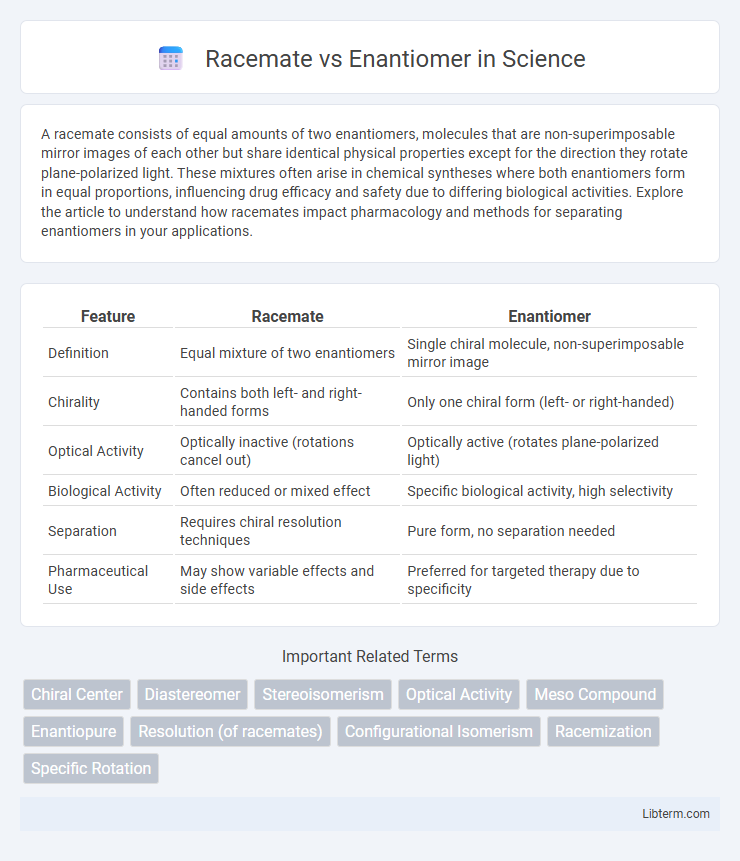
| Feature | Racemate | Enantiomer |
|---|---|---|
| Definition | Equal mixture of two enantiomers | Single chiral molecule, non-superimposable mirror image |
| Chirality | Contains both left- and right-handed forms | Only one chiral form (left- or right-handed) |
| Optical Activity | Optically inactive (rotations cancel out) | Optically active (rotates plane-polarized light) |
| Biological Activity | Often reduced or mixed effect | Specific biological activity, high selectivity |
| Separation | Requires chiral resolution techniques | Pure form, no separation needed |
| Pharmaceutical Use | May show variable effects and side effects | Preferred for targeted therapy due to specificity |
Introduction to Chirality in Chemistry
Chirality in chemistry refers to molecules that exist in two non-superimposable mirror image forms called enantiomers, each exhibiting distinct spatial arrangements affecting their chemical behavior. A racemate is a mixture containing equal amounts of both enantiomers, resulting in no optical activity due to their opposing rotations of plane-polarized light. Understanding chirality and the differences between racemates and enantiomers is crucial in fields such as pharmaceuticals, where enantiomeric purity influences drug efficacy and safety.
Defining Enantiomers: Mirror-Image Molecules
Enantiomers are chiral molecules that exist as non-superimposable mirror images of each other, characterized by identical physical properties except for their interaction with plane-polarized light and chiral environments. Each enantiomer possesses opposite optical activities, rotating plane-polarized light in equal magnitude but opposite directions, designated as dextrorotary (D-) or levorotary (L-). Racemates, or racemic mixtures, contain equal amounts of both enantiomers, resulting in optical inactivity due to mutual cancellation of their optical rotations.
What is a Racemate?
A racemate is a mixture containing equal amounts of two enantiomers, which are molecules that are non-superimposable mirror images of each other. Unlike a single enantiomer, a racemate exhibits no optical activity because the optical rotations of the enantiomers cancel each other out. Racemates are significant in pharmaceuticals since the biological activity of each enantiomer can differ, affecting efficacy and safety.
Structural Differences: Racemate vs Enantiomer
Racemates consist of an equal mixture of two enantiomers, each being non-superimposable mirror images of one another with identical physical and chemical properties except for optical activity. Enantiomers differ in the spatial arrangement of atoms around a chiral center, causing opposite optical rotation but the same connectivity and molecular formula. The structural difference lies in the three-dimensional configuration, where racemates contain both configurations in a 1:1 ratio, neutralizing optical activity, while individual enantiomers exhibit distinct chirality and rotate plane-polarized light in opposite directions.
Synthesis and Production Methods
Racemates are synthesized through non-stereoselective methods, often yielding an equal mixture of enantiomers during chemical reactions without chiral catalysts or directing agents. Enantiomerically pure compounds are typically produced using stereoselective synthesis techniques, such as asymmetric catalysis, chiral auxiliaries, or biocatalysis, which favor the formation of a single enantiomer. Resolution methods, including chiral chromatography or crystallization, are also employed post-synthesis to separate enantiomers from racemic mixtures, enhancing the production of enantiomerically enriched substances.
Physical and Chemical Properties Comparison
Racemates contain equal amounts of two enantiomers, resulting in no optical activity, whereas pure enantiomers rotate plane-polarized light either clockwise or counterclockwise. While enantiomers share identical melting points, boiling points, and solubilities in achiral environments, racemates often exhibit different physical properties such as melting points and solubility due to their distinct crystal packing. Chemically, enantiomers react identically with achiral reagents but show different reaction rates and product distributions when interacting with chiral agents or biological systems, a distinction not present in racemates as they contain both mirror-image forms.
Optical Activity: Racemates vs Enantiomers
Racemates contain equal amounts of left- and right-handed enantiomers, resulting in no net optical activity because their rotations cancel each other out. In contrast, enantiomers are optically active substances that rotate plane-polarized light in opposite directions, with one being dextrorotatory (d-) and the other levorotatory (l-). The measurement of optical rotation using a polarimeter distinguishes enantiomers, while racemates exhibit zero optical rotation due to their balanced chiral composition.
Pharmaceutical Implications and Drug Efficacy
Racemates contain equal parts of two enantiomers, which can result in mixed pharmacological effects and variability in drug efficacy compared to a single enantiomer. Enantiomers often exhibit distinct interactions with biological targets due to their stereochemistry, influencing drug potency, safety, and metabolism. Pharmaceutical development increasingly favors enantiomerically pure compounds to optimize therapeutic outcomes and minimize adverse effects.
Analytical Techniques for Differentiation
Chiral chromatography, such as high-performance liquid chromatography (HPLC) with chiral stationary phases, effectively separates racemates into individual enantiomers based on differential interactions. Nuclear magnetic resonance (NMR) spectroscopy using chiral shift reagents provides distinct spectral differences that identify enantiomeric configurations within racemic mixtures. Circular dichroism (CD) spectroscopy measures differential absorption of left- and right-circularly polarized light, enabling precise differentiation between enantiomers in racemates.
Conclusion: Importance in Chemical and Pharmaceutical Sciences
Racemates and enantiomers play critical roles in chemical and pharmaceutical sciences due to their distinct stereochemical properties and biological activities. Understanding the differences between racemates, which contain equal amounts of both enantiomers, and pure enantiomers is essential for drug efficacy, safety, and regulatory approval. The precise control and characterization of these stereoisomers directly impact the development of targeted therapies and the optimization of pharmacokinetic and pharmacodynamic profiles.
Racemate Infographic
 libterm.com
libterm.com